X-RAYS
– electromagnetic radiation with wavelengths 10–4 – 10 A (10–5 – 1 nm).
Also on the topic:
ELECTROMAGNETIC RADIATION
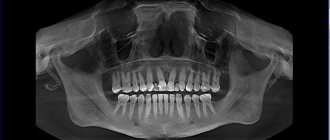
In 1895, the German physicist Roentgen, conducting experiments on the passage of current between two electrodes in a vacuum, discovered that a screen covered with a luminescent substance (barium salt) glows, although the discharge tube is covered with a black cardboard screen - this is how radiation penetrating through opaque barriers, called X-rays X-rays. It was discovered that X-ray radiation, invisible to humans, is absorbed in opaque objects the more strongly, the higher the atomic number (density) of the barrier, so X-rays easily pass through the soft tissues of the human body, but are retained by the bones of the skeleton. Sources of powerful X-rays have been designed to make it possible to illuminate metal parts and find internal defects in them.
The German physicist Laue suggested that X-rays are the same electromagnetic radiation as visible light rays, but with a shorter wavelength and all the laws of optics apply to them, including the possibility of diffraction. In visible light optics, diffraction at an elementary level can be represented as the reflection of light from a system of lines - a diffraction grating, which occurs only at certain angles, and the angle of reflection of the rays is related to the angle of incidence, the distance between the lines of the diffraction grating and the wavelength of the incident radiation. For diffraction to occur, the distance between the lines must be approximately equal to the wavelength of the incident light.
Laue suggested that X-rays have a wavelength close to the distance between individual atoms in crystals, i.e. the atoms in the crystal create a diffraction grating for x-rays. X-rays directed at the surface of the crystal were reflected onto the photographic plate, as predicted by theory.
Any changes in the position of atoms affect the diffraction pattern, and by studying X-ray diffraction, one can find out the arrangement of atoms in a crystal and the change in this arrangement under any physical, chemical and mechanical influences on the crystal.
Nowadays, X-ray analysis is used in many fields of science and technology; with its help, the arrangement of atoms in existing materials has been determined and new materials have been created with a given structure and properties. Recent advances in this field (nanomaterials, amorphous metals, composite materials) create a field of activity for the next scientific generations.
Occurrence and properties of X-ray radiation
The source of X-rays is an X-ray tube, which has two electrodes - a cathode and an anode. When the cathode is heated, electron emission occurs; electrons escaping from the cathode are accelerated by the electric field and strike the surface of the anode. What distinguishes an X-ray tube from a conventional radio tube (diode) is mainly its higher accelerating voltage (more than 1 kV).
When an electron leaves the cathode, the electric field forces it to fly towards the anode, while its speed continuously increases; the electron carries a magnetic field, the strength of which increases with increasing speed of the electron. Reaching the anode surface, the electron is sharply decelerated, and an electromagnetic pulse with wavelengths in a certain interval appears (bremsstrahlung). The distribution of radiation intensity over wavelengths depends on the anode material of the X-ray tube and the applied voltage, while on the short wave side this curve begins with a certain threshold minimum wavelength, depending on the applied voltage. The combination of rays with all possible wavelengths forms a continuous spectrum, and the wavelength corresponding to the maximum intensity is 1.5 times the minimum wavelength.
As the voltage increases, the X-ray spectrum changes dramatically due to the interaction of atoms with high-energy electrons and quanta of primary X-rays. An atom contains internal electron shells (energy levels), the number of which depends on the atomic number (denoted by the letters K, L, M, etc.) Electrons and primary X-rays knock electrons out of one energy level to another. A metastable state arises and for the transition to a stable state a jump of electrons in the opposite direction is necessary. This jump is accompanied by the release of an energy quantum and the appearance of X-ray radiation. Unlike X-rays with a continuous spectrum, this radiation has a very narrow range of wavelengths and high intensity (characteristic of radiation) ( see
. rice.). The number of atoms that determine the intensity of the characteristic radiation is very large; for example, for an X-ray tube with a copper anode at a voltage of 1 kV and a current of 15 mA, 1014–1015 atoms produce characteristic radiation in 1 s. This value is calculated as the ratio of the total power of X-ray radiation to the energy of an X-ray quantum from the K-shell (K-series of X-ray characteristic radiation). The total power of X-ray radiation is only 0.1% of the power consumption, the rest is lost mainly due to conversion to heat.
Due to their high intensity and narrow wavelength range, characteristic X-rays are the main type of radiation used in scientific research and process control. Simultaneously with the K-series rays, L and M-series rays are generated, which have significantly longer wavelengths, but their use is limited. The K-series has two components with close wavelengths a and b, while the intensity of the b-component is 5 times less than a. In turn, the a-component is characterized by two very close wavelengths, the intensity of one of which is 2 times greater than the other. To obtain radiation with one wavelength (monochromatic radiation), special methods have been developed that use the dependence of absorption and diffraction of x-rays on wavelength. An increase in the atomic number of an element is associated with a change in the characteristics of the electron shells, and the higher the atomic number of the X-ray tube anode material, the shorter the K-series wavelength. The most widely used are tubes with anodes made of elements with atomic numbers from 24 to 42 (Cr, Fe, Co, Cu, Mo) and wavelengths from 2.29 to 0.712 A (0.229 - 0.712 nm).
In addition to the X-ray tube, sources of X-ray radiation can be radioactive isotopes, some can directly emit X-rays, others emit electrons and a-particles that generate X-rays when bombarding metal targets. The intensity of X-ray radiation from radioactive sources is usually much less than that of an X-ray tube (with the exception of radioactive cobalt, which is used in flaw detection and produces radiation of a very short wavelength - g-radiation), they are small in size and do not require electricity. Synchrotron X-rays are produced in electron accelerators; the wavelength of this radiation is significantly longer than that obtained in X-ray tubes (soft X-rays), and its intensity is several orders of magnitude higher than the radiation intensity of X-ray tubes. There are also natural sources of X-ray radiation. Radioactive impurities have been found in many minerals, and X-ray emission from space objects, including stars, has been recorded.
History of radiography
X-ray images were discovered in 1895 by Wilhelm Conrad Roentgen (1845-1923), a professor at the University of Würzburg in Germany.

While working with a cathode ray tube in his laboratory, Roentgen observed the fluorescent glow of crystals on the table near his tube.
Did you know: That the tube with which Roentgen worked consisted of a glass shell (bulb) with positive and negative electrodes encapsulated in it. The air in the tube was pumped out and when high voltage was applied, the tube produced a fluorescent glow. X-ray covered the tube with heavy black paper and discovered green fluorescent light generated by the material located several feet from the tube.
He concluded that a new type of beam was being emitted from the tube. This beam was able to pass through thick paper coverings and excite phosphorescent materials in the room. He discovered that the new beam could pass through most substances that cast shadows from solid objects. X-ray also discovered that the beam could pass through human tissue, but not through bones or metal objects.

One of Roentgen's first experiments, at the end of 1895, was a photograph of his wife Bertha's hand.
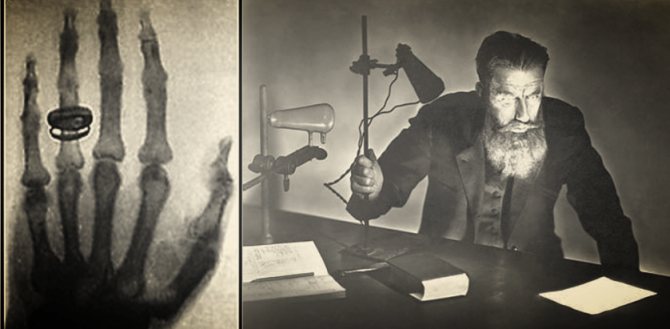
Interestingly, the first use of X-rays was for industrial (not medical) applications.
Check out these interesting photos
X-ray image of a baby elephant
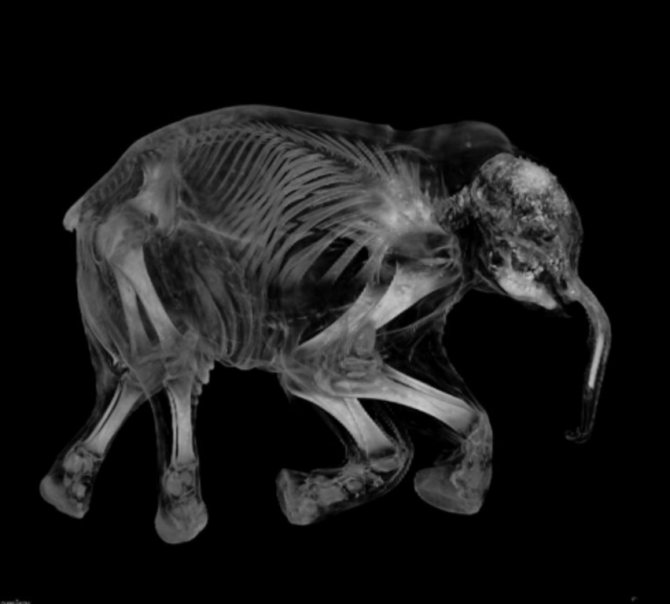
Image 1 of 27
Roentgen's discovery was a scientific bombshell and was greeted with extraordinary interest by scientists and people alike. Scientists everywhere could repeat his experiment because the cathode tube was very well known during this period. Many scientists abandoned other areas of research to work with the mysterious rays.
Newspapers and magazines of the time provided the public with many stories, some true, others bizarre, but all about the properties of the newly discovered rays.
The public imagination was occupied by this invisible ray, capable of passing through solid matter and, together with a photographic plate, creating an image of bones and internal parts of the body. The scientific imagination was captured by the demonstration of wavelengths shorter than light. This gave rise to new possibilities in physics and began to be used to study the structure of matter.
Much enthusiasm has been shown for the potential use of rays as an aid in medicine and surgery. Within a month of the discovery being announced, several medical X-rays were taken in Europe and the United States and were used by surgeons to guide their work.
In June 1896, just 6 months after Roentgen announced his discovery, military doctors began using X-rays to detect bullets in wounded soldiers.
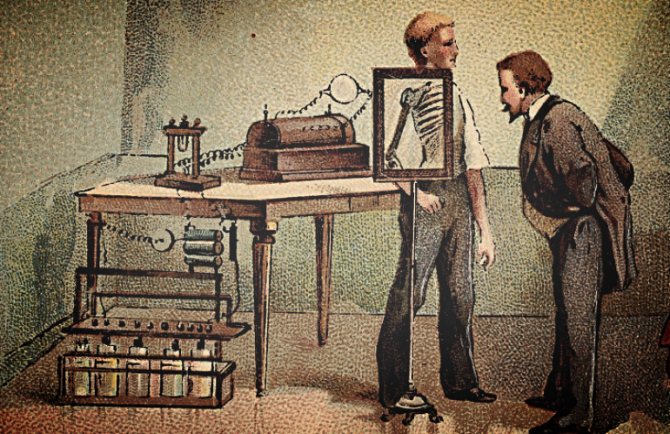
Before 1912, X-rays were used little outside the sciences of medicine and dentistry , although some X-rays of metals were obtained. The reason X-rays were not used industrially before this date was that X-ray tubes (the source of the X-rays) broke down at the voltages required to produce rays of satisfactory penetration for industrial purposes.
However, this changed in 1913 when high-vacuum X-ray tubes developed by Coolidge became available. The high-vacuum tubes were an intense and reliable source of X-rays, operating at energies up to 100,000 volts.
In 1922, industrial radiography took another step forward with the advent of the 200,000-volt X-ray tube, which made it possible to produce radiographs of thick steel parts in a reasonable time. In 1931, General Electric developed 1,000,000-volt X-ray generators that became effective tools for industrial radiography. That same year, the American Society of Mechanical Engineers allowed x-ray approval of welded pressure vessels, further opening the door to industrial acceptance and use.
Interaction of X-rays with crystals
In X-ray studies of materials with a crystalline structure, interference patterns resulting from the scattering of X-rays by electrons belonging to the atoms of the crystal lattice are analyzed. Atoms are considered immobile, their thermal vibrations are not taken into account, and all electrons of the same atom are considered concentrated at one point - a node of the crystal lattice.
To derive the basic equations for X-ray diffraction in a crystal, the interference of rays scattered by atoms located along a straight line in the crystal lattice is considered. A plane wave of monochromatic X-ray radiation falls on these atoms at an angle whose cosine is equal to a0. The laws of interference of rays scattered by atoms are similar to those existing for a diffraction grating, which scatters light radiation in the visible wavelength range. In order for the amplitudes of all vibrations to add up at a large distance from the atomic row, it is necessary and sufficient that the difference in the paths of the rays coming from each pair of neighboring atoms contains an integer number of wavelengths. At a distance between atoms a
this condition looks like:
A
(a
–
a0)
= h
l,
where a is the cosine of the angle between the atomic row and the deflected beam, h is
integer. In all directions that do not satisfy this equation, the rays do not propagate. Thus, scattered rays form a system of coaxial cones, the common axis of which is the atomic row. Traces of cones on a plane parallel to the atomic row are hyperbolas, and on a plane perpendicular to the row they are circles.
When rays are incident at a constant angle, polychromatic (white) radiation is decomposed into a spectrum of rays deflected at fixed angles. Thus, the atomic series is a spectrograph for x-rays.
Generalization to a two-dimensional (flat) atomic lattice, and then to a three-dimensional volumetric (spatial) crystal lattice gives two more similar equations, which include the angles of incidence and reflection of X-ray radiation and the distances between atoms in three directions. These equations are called Laue's equations and form the basis of X-ray diffraction analysis.
The amplitudes of rays reflected from parallel atomic planes add up, etc. the number of atoms is very large, the reflected radiation can be detected experimentally. The reflection condition is described by the Wulff–Bragg equation 2d sinq = nl, where d is the distance between adjacent atomic planes, q is the grazing angle between the direction of the incident beam and these planes in the crystal, l is the x-ray wavelength, n is an integer called the order of reflection . Angle q is the angle of incidence with respect specifically to atomic planes, which do not necessarily coincide in direction with the surface of the sample under study.
Several methods of X-ray diffraction analysis have been developed, using both radiation with a continuous spectrum and monochromatic radiation. The object under study can be stationary or rotating, can consist of one crystal (single crystal) or many (polycrystal); diffracted radiation can be recorded using a flat or cylindrical X-ray film or an X-ray detector moving around the circumference, but in all cases during the experiment and interpretation of the results, the Wulff–Bragg equation is used.