16 900
30 000
Intimate plastic surgery with a 43% discount
Medline-Service offers intimate plastic surgery of the labia majora or minora for 16,900 rubles. instead of 30,000 rub.
Terms and prices
17 000
26 440
“Happy Parents” program with a 35% discount
"Medline-Service" offers the "Happy Parents" program with a 35% discount
Terms and prices
95 000
118 970
Prenatal care program “Standard +”
“Medline-Service” offers the “Standard +” prenatal care program (pregnancy management) for 95,000 rubles. instead of 118,970 rub.
Terms and prices
66 000
83 180
Prenatal care program "Standard"
“Medline-Service” offers the “Standard” prenatal care program (pregnancy management) for 66,000 rubles. instead of 83,180 rub.
Terms and prices
5 500
8 820
Express diagnostics – Check-UP “Women’s health” with a 35% discount
"Medline-Service" offers express diagnostics - Check-UP "Women's Health" with a 35% discount
Terms and prices
Follicles are the structural components of the gonads in women. One of these elements, called dominant, during ovulation releases an egg that is ripe for conception. With the normal structure of the follicle, as well as its timely ripening, a woman is able to become pregnant. Any deviations from the norm can lead to the development of a cyst on the ovary or even infertility. There are many reasons that can cause these disorders, so if you experience any unusual symptoms, you should immediately seek medical help.
Functions and purpose of follicles
A follicle is an immature egg that is surrounded by a layer of epithelial cells and a double layer of connective tissue. Its main task is to protect the reproductive cell from the negative effects of various factors. It is on these elements that the correct maturation of the egg and its fertilization, and, consequently, the ability to become pregnant and bear a child depend. They are also designed to produce the female hormone estrogen.
In women, the reproductive system and follicular apparatus develop in the perinatal period, and at this stage a constant number of follicles is established, which remains unchanged throughout life (30 - 50 thousand).
There are several stages of follicle development in the ovaries:
- The formation of several small immature cells.
- On day 5, ultrasound allows you to see up to ten antral follicles on the periphery of the ovary (their size ranges from 2 to 4 mm).
- After seven days, their size increases to 6 mm, which makes it possible to examine the network of capillaries at their base.
- On the eighth day, dominant follicles are determined, which continue to grow and develop.
- Around day 10, ultrasound makes it possible to identify the dominant follicle, which has the largest size (about 15 mm), while the rest will be half the size.
- After two weeks, the dominant reaches a size of 25 mm. At this moment, the active production of the female hormone estrogen occurs, under the influence of which the protective membrane breaks through (allowing the egg to come out) and ovulation occurs on days 15–16.
- The egg enters the fallopian tubes. As a result of its meeting with the sperm, fertilization occurs. Otherwise, during menstruation, it leaves the uterus along with the epithelium.
Maturation in each menstrual cycle
From the beginning of the menstrual cycle, about 8-10 secondary follicles are formed in both ovaries. From about the eighth or ninth day of the cycle, the bubbles begin to fill with liquid formed under the influence of estrogen synthesized by the female body. And already at this stage the dominant follicle is noticeable: it is larger than the others, and this can be seen on an ultrasound.
Release of the egg from the follicle.
The bubble continues to fill with fluid, stretches and bursts at the moment of ovulation. A mature egg is released, which will begin to move along the fallopian tube into the uterus to connect with the sperm. On what day does the breakup occur? This depends on the duration of the menstrual cycle: if it lasts 28-30 days, then ovulation and, accordingly, the release of the egg from the burst follicle occurs on the 14-16th day (counting from the beginning of menstruation).
In place of the ruptured vesicle, a corpus luteum is formed - a temporary endocrine gland that actively synthesizes progesterone and prepares the uterus for a possible pregnancy. The hormone produced makes the endometrium loose and soft so that the fertilized egg can firmly establish itself in it and begin to develop.
Violations of the norm
What is the norm of follicles in the ovary? An excess or deficiency of these elements is a violation. If there are more than ten immature eggs in one ovary and this figure remains unchanged throughout the entire cycle, we can talk about exceeding the norm (this can only be detected by ultrasound). Depending on the number of elements detected during ultrasound examination, the following conclusion can be made:
- in the range from seven to sixteen – there are many follicles in the ovary and the probability of conception is high;
- from four to six – low probability of pregnancy;
- less than four – the probability of conception is practically absent.
However, this does not always indicate any pathology and can be caused by stress, anxiety or overwork. In this case, the number of follicles in the ovaries is normalized after the first ovulation. Therapy is carried out if disorders are caused by the following reasons:
- incorrect choice of contraceptives;
- dysfunction of the thyroid gland;
- rapid weight gain or loss;
- failure of the endocrine system;
- increased levels of prolactin in the body.
The above disorders can be identified using diagnostic procedures.
An insufficient number or absence of germ cells can be caused by hormonal imbalance or early menopause. Typically, this problem is monitored on the seventh day of the menstrual cycle. In this case, hormonal drugs are prescribed to treat the woman’s reproductive function.
To summarize, we can distinguish two existing options for follicle development:
- The course of the menstrual cycle with one dominant in the left or right ovary.
- Its absence, as a result, the egg does not mature, and the menstrual cycle is disrupted. In this case, conception is impossible.
Possible pathologies
Let's look at some deviations from the norm:
- There is no dominant follicle. This suggests that there will most likely not be ovulation in the current menstrual cycle. Every healthy woman experiences anovulatory cycles once or twice a year. If you don't ovulate for several months in a row, this is not normal.
- Multiple follicles or so-called multifollicular ovaries are a deviation that develops as a result of hormonal disorders. The dominant follicle may be absent or develop slowly, which will reduce the chance of conception.
- Cyst formation. The dominant follicle does not burst, fills with fluid and stretches, forming a benign formation - a cyst (it can grow or regress on its own, that is, burst and disappear).
- Atresia is a slowdown, stopping the growth of the main vesicle and its subsequent death without the release of a mature egg.
- Persistence. The dominant follicle reaches the desired size, but does not rupture and remains unchanged until the onset of menstruation. Conception becomes impossible.
- Luteinization. The corpus luteum begins to form when there is a whole follicle in the ovary.
The listed pathologies are noticeable on ultrasound and are caused by hormonal imbalances or diseases of the reproductive system.
A dominant follicle is necessary for fertilization. But conception will occur if the vesicle is formed correctly and a mature egg is released from it. The information presented in the article will help you understand the mechanism of fertilization and identify some problems.
(voted: 5, rating: 4.80 out of 5)
Share the news on social networks
Ask a Question! You have questions? Feel free to ask any questions! And our staff specialist will help you. Go>>
Tags: follicle
- Recommended Articles
- Planning pregnancy with chronic endometritis
- Dangerous days for conception
- Cervical erosion during pregnancy planning
« Previous entry
Dominant follicle
In the middle of the cycle, several follicles usually mature, and the rest dissolve. The largest and most developed protective element is dominant. It protects the egg ready for fertilization. Directly during the period of ovulation in the right or left ovary, it can reach a size of several centimeters. Under the influence of hormones, it ruptures, as a result of which the egg is released and rushes to the fallopian tubes, which means that the possibility of pregnancy arises.
In rare cases, simultaneous maturation of dominants occurs in both ovaries. This makes it possible to conceive twins.
There is also a reserve of follicles that are ready for fertilization. They are called antral. When preparing for in vitro fertilization, specialists determine how many follicles are formed and, based on this data, make predictions about the likelihood of pregnancy.
Stages of development
Over the entire period of a woman’s life, starting from birth, follicles go through several stages of development:
- Primordial stage. These are immature follicular cells that are formed during the formation of a female fetus. They are very small and do not exceed 0.05 millimeters in diameter. Follicles capable of reproducing by division are covered with epithelium and move to the next stage.
- Primary or preantral formations reach 0.2 mm in diameter. During active puberty of a girl, the pituitary gland actively synthesizes folliculotropin, which accelerates the development of cells, strengthens their membranes and forms a protective layer.
- Secondary or antral follicles increase in size to 0.5 mm. Their total number is about 8-10. Under the influence of estrogen, the internal cavity begins to fill with liquid, which stretches the walls and provokes the rapid growth of bubbles. Secondary follicles, by the way, are considered temporary organs of the endocrine system that produce hormones.
- As a rule, only one follicular formation passes into the next stage - the dominant one. It becomes the most voluminous and contains an egg that is almost completely mature and ready for fertilization. The vesicle consists of a large number of granulosa cells and is designed to provide reliable protection for the oocyte until ovulation. The remaining secondary follicles at this time synthesize estrogens, which ensure the rapid development of the main vesicle.
- The tertiary or preovulatory vesicle is called a graafian vesicle. Follicular fluid completely fills its cavity, its volume increases a hundred times compared to the original. During ovulation, the sac ruptures and an egg is released.
Violation of follicular development
Any disturbances in the development of follicles lead to serious consequences, including infertility. The following deviations may contribute to this:
- follicular ovaries;
- inflammatory processes of the pelvic organs;
- insufficient production of the female hormone - estrogen;
- endocrine system disorders;
- problems with ovulation;
- dysfunction of the pituitary gland;
- premature menopause (surgical or natural);
- stress, depression, nervous tension.
Also a very important point is the state of the dominant follicle, which may be absent, not reach the required size, be late in maturation, or not develop at all.
Follicular apparatus disorders
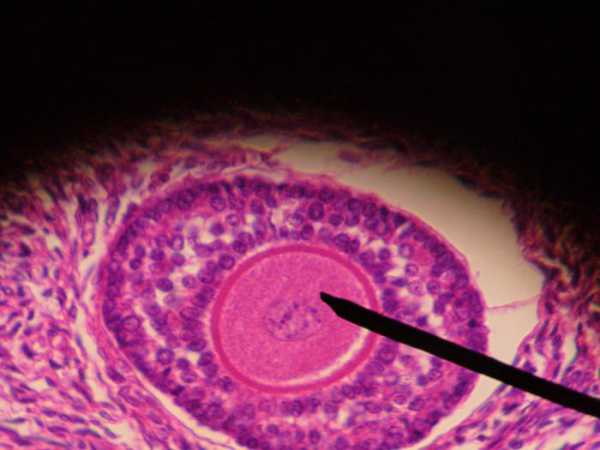
One of the common pathologies is the maturation of a large number of follicles, this condition is called polycystic ovary syndrome. With this pathology, the dominant follicle does not mature, so it is very difficult for such women to become pregnant. This pathology requires long-term hormonal treatment. It happens when few follicles are produced, or they do not mature at all. This occurs due to hormonal imbalance when the body has low levels of estrogen.
Persistent follicles
With age-related changes or in adolescence, a disruption of the activity of the follicular apparatus, called persistence, often occurs. The main symptoms of the disease are menstrual irregularities, heavy menstrual flow, and bleeding. In this case, reverse development of the follicle in the ovaries occurs, which can lead to the formation of a cyst. To prevent the cyst from bursting, hormonal therapy is prescribed. If the tumor size is significant, hormonal treatment is ineffective and surgery is required. The phenomenon of persistence is accompanied by:
- hormonal disorders;
- thickening of the endometrial mucosa;
- endometrial rejection;
- compression of the uterus;
- pain in the lower abdomen, accompanied by spotting or bleeding.
Follicles grow poorly
Underdevelopment of the structural components of the ovaries is one of the main causes of female infertility. In the absence of a dominant follicle, luteinizing hormones do not enter the bloodstream, stimulating the onset of ovulation. The main causes of insufficient development of germ cells include:
- pathologies of the hypothalamus;
- violation of the generative function of the ovaries;
- contraceptive abuse;
- consequences of infection in the small genital organs;
- pathologies of the thyroid gland;
- endocrine disorders;
- depression and emotional instability;
- formation of tumors in the pituitary gland.
Adequate hormonal therapy allows you to restore the menstrual cycle and the process of oocyte maturation. If conservative treatment is ineffective, ovarian cauterization is prescribed, which consists of surgical removal of underdeveloped cells from the gonads.
Primordial folliculosis
The ovarian reserve (the supply of eggs in women) is formed in the womb. The primary stage of development of the protective follicle is primordial. In this case, the egg primordia are located on the inner surface of the ovaries and are protected by granulosa cells. This picture is observed until the onset of menstruation. Puberty is characterized by:
- production of a hormone that stimulates follicle development:
- growth of the egg nucleus under the influence of follicle-stimulating hormone;
- maturation of the protective shell of the egg;
- the monthly development of several follicles that protect the reproductive cell.
Folliculometry
Ultrasound diagnostics is carried out to determine the number of follicles, the stage of their development and growth.
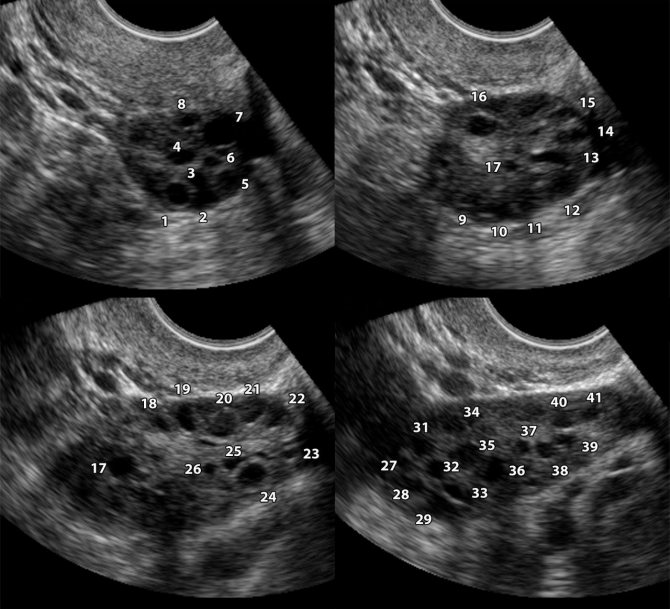
Polycystic disease in folliculometry images
But this study cannot be carried out on any day; for correct diagnosis, this is done on a certain day of the menstrual cycle. Because it is simply impossible to determine their presence during the menstrual week. But on days 8-9 you can clearly see the presence of small “bubbles”.
Such a study makes it possible to determine whether there are dominant follicles, while normally only one develops in one of the ovaries during 1 cycle. But there are times when several of them ripen. This increases the likelihood of conceiving a child and having multiple pregnancies.
The dominant follicle on ultrasound is found by its rounded shape and increased size; the normal size in a mature state is up to 20-25 mm.
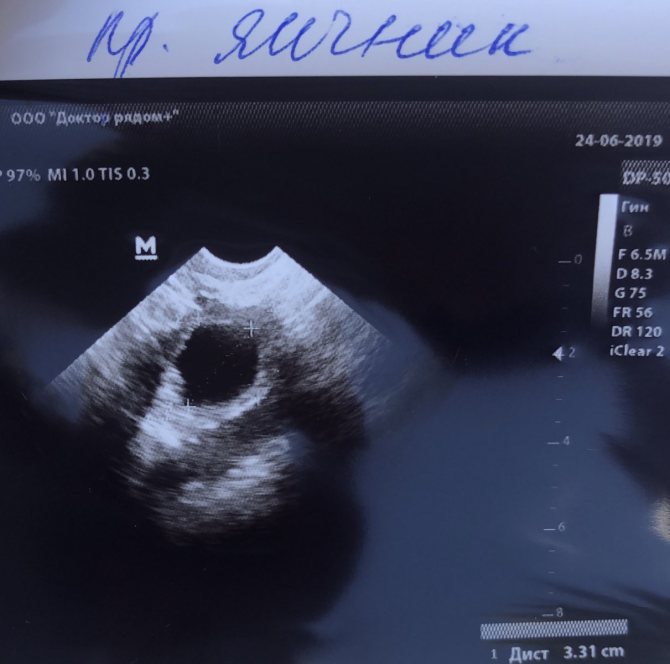
Dominant follicle on folliculometry
Antral folliculosis
Antral follicles in the ovaries are no more than 8 mm in size. They develop on the seventh – eighth day of the menstrual cycle. Monitoring their number in women is important at the stage of determining her ability to become pregnant artificially. The number of protective elements can be determined by ultrasound and, based on the data obtained, the reserve of eggs capable of fertilization can be determined.
If the antral elements reach a size of up to 5 mm, the likelihood of pregnancy is low. If the follicle size is 5–8 mm, a woman is likely to become pregnant without the help of doctors. It is worth noting that during pregnancy, follicles do not form in the ovaries.
Goals and advantages of the method
Ultrasound examination of folliculogenesis is indicated for women in order to identify possible pathologies in the process of egg maturation, as well as to control the quality of biomaterial before its collection for ART. The study allows you to observe the growth of the dominant follicle and determine its readiness for fertilization.
Diagnostics allows you to determine:
- The quality of the ovaries and the state of the woman’s reproductive system as a whole;
- Endometrial condition;
- Identify the possible cause of infertility;
- Exact day of the cycle;
- Factors influencing the disruption of the menstrual cycle;
- The optimal day for follicle puncture is important for women participating in IVF programs or for oocyte donors.
Control of folliculogenesis, as a diagnostic method, has the following advantages:
- It is absolutely harmless and painless (like a regular pelvic ultrasound).
- It can be performed in a transvaginal or transabdominal format.
- It is distinguished by high accuracy of the results obtained.
- Allows you to increase the chances of successful IVF or correctly determine the cause of pathologies in the reproductive system of the female body.
- It has an objectively affordable price.
Preovulatory follicle
At the last stage of its maturation, the egg is ready for fertilization. In this case, the follicle is almost completely filled with fluid, the day before ovulation, estrogen production increases and the following phenomena are observed:
- stimulates the release of lutein, which promotes the onset of ovulation;
- the preovulatory follicle forms a protrusion on its wall, at the site of which a breakthrough later occurs (ovulation);
- after ovulation, the production of progesterone is activated, preventing endometrial rejection;
- a corpus luteum is formed, which subsequently forms a network of blood vessels and contributes to the appearance and development of the placenta.
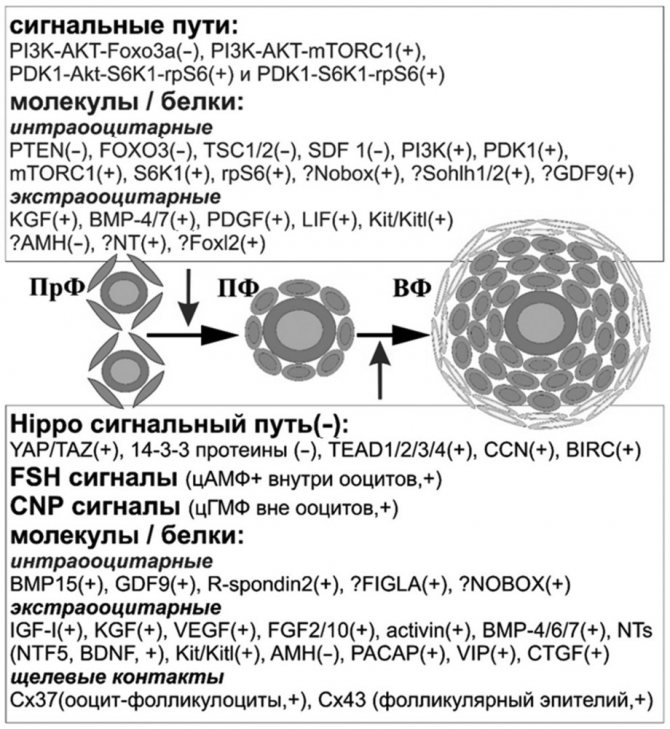
— potential participation (compiled by the author). Continuing the discussion of the processes of folliculogenesis, attention should be paid to the main and potential factors and signaling pathways involved in the process of early follicular development (see diagram).
Initiation of follicular growth
. Primordial follicles are the fundamental reproductive units of the ovary. The transition from the resting state to the growth stage is called recruitment, or activation. Histologically, the primordial follicle contains a small primary oocyte (~25 µm in diameter) in prophase I of meiosis, surrounded by a single layer of flattened follicular cells closely associated with the oocyte and basement membrane. Primordial follicles are located in a microenvironment that excludes contact with most other cells and blood vessels and have limited access to the endocrine system. Histological signs of activation of dormant follicles are the acquisition of mitotic potential by follicular epithelial cells (change in shape to cubic). The process is accompanied by oocyte growth [1].
Recruitment of primordial follicles is primarily controlled by a paracrine interaction between the oocyte, follicular epithelium, and adjacent thecal and interstitial cells. The pool of resting follicles is under strict control of inhibitory factors; activation occurs with increasing stimulating influence. The sequence of actions is largely mediated by secreted growth factors, including several members of the transforming growth factor-beta (TGF-β) superfamily [2, 3].
Experimental data suggest that not only external stimuli, but also the oocytes themselves largely control the transmission of signals that regulate the recruitment and growth rate of follicles. The main signaling pathways pass through PI3K (phosphatidylinositol 3-kinase) [4].
First, PI3K-AKT-Foxo3a signaling is required to inhibit follicle activation [1, 3, 5]. This signaling pathway is mediated by PTEN (phosphotensin) [6]. The absence of PTEN activates PI3K to produce the phospholipid phosphatidylinositol-3, -4, -5-triphosphate (PIP3) and Akt (protein kinase B) signaling. PTEN-deficient mice (OoPten –/–) show overactivation of primordial follicles (growth reaches the primary and secondary stages) and early ovarian depletion [7]. The Foxo3a (for khead box O3A) protein, which belongs to the for khead family of transcription factors, also inhibits recruitment. Foxo3a is found in the nuclei of oocytes and follicular epithelial cells of primordial follicles. In growing follicles, factor expression decreases. Foxo3a knockout mice (Foxo3a –/–) exhibit a phenotype similar to OoPten –/–, accompanied by oocyte growth and follicle activation [5]. Depletion of the primordial follicle pool in Foxo3a –/– leads to infertility. In OoPten –/– animals, constant Akt activation suppresses Foxo3a, so PTEN is located upstream of FOXO3 in this signaling pathway [7].
The second signaling pathway is PI3K-AKT-mTORС1 (protein kinase-serine-threonine specificity) [8]. The multimolecular signaling complex TORC1 (mammalian target of rapamycin) is controlled by heterodimeric proteins: TSC1 (hamartin) and TSC2 (tuberin) [1, 3]. TSC1- and TSC2-deficient mice (OoTsc1 –/–, OoTsc2 –/–) exhibit premature activation of primordial follicles due to increased S6K1-rpS6 (S6 kinase 1 and ribosomal protein S6) signaling. The phenotype is also similar to OoPten –/– [9]. Both proteins (TSC and PTEN) regulate follicle recruitment via the S6K1-rpS6 pathway. TSC inhibits the phosphorylation of S6K1 to threonine 389, PTEN to threonine 229. Thus, intracellular (intraoocyte) signaling that maintains the “rest” of primordial follicles is provided by three main factors: PTEN, FOXO3 and TSC.
PI3K activation is also due to extracellular signals. Paracrine signaling between the oocyte and somatic cells of the environment is provided by the main ligand receptor - RPTK (receptor tyrosine kinase). In postnatal ovaries, Kit (stem cell factor) is expressed on the surface of oocytes, and Kitl (ligand) is found in pregranulosa cells and follicular epithelial cells of all types of growing follicles [10].
During early stages of follicular development, in which FSH receptors are absent, PI3K signaling is controlled by Kit/Kitl signaling. In primary oocytes, Kitl leads to phosphorylation of Akt and Foxo3a. In mammals, Akt inactivates Foxo3a through extracellular signaling involving growth factors and insulin. The effect of the latter on activation of primordial follicles is the inhibition of FOXO3. In the PI3K signaling pathway, PIP2 (phosphatidylinositol 4,5-bisphosphate) is phosphorylated to PIP3. The factor is a second messenger for PDK1 (3-phosphoinositide-dependent kinase-1) and Akt. The latter contributes to the life support of cells, causes the growth and activation of oocytes. In mice with oocyte-specific exclusion of the gene encoding PDK1 (OoPDK1 –/–), suppression of PDK1-Akt-S6K1-rpS6 signals leads to depletion of the primordial follicle pool [7]. In contrast, PTEN inhibits PI3K by converting PIP3 to PIP2. The main target of Akt is the FOXO family, which suppresses the activation of primordial follicles and maintains follicles in a dormant state, stimulating proapoptotic genes. However, in double knockouts (OoPten –/–, OoPDK1 –/–), deactivation of rpS6 phosphorylation prevents excessive follicular activation, in contrast to that in isolated PDK1 deletion (OoPDK1 –/–) [7].
In addition to the two signaling pathways, a chemokine of the CXC subfamily, probably secreted by oocytes, SDF1 (stromal cell factor 1), has an auto-, paracrine effect that inhibits follicle activation. CXCL12 and its receptor, CXCR4, are involved in various physiological processes, including the migration of primordial germ cells of the gonads. SDF1α expression increases during follicular development, and in vitro
with factor inhibitor (AMD3100) keeps follicles in an inactive state, so SDF1/CXCR4 signaling may play an important role in maintaining the pool of primordial follicles [11].
Recruitment of primordial follicles also involves factors outside the direct interaction of the oocyte-folliculocyte complex. The stromal interstitial cells surrounding the primordial follicle secrete KGF (keratinocyte growth factor), BMP4 and BMP7 (bone morphogenetic proteins 4 and 7), PDGF (platelet-derived growth factor), LIF (leukemia inhibitory factor). Factors contribute to the transition of primordial follicles to the growing pool [1, 10]. BMP4 and BMP7 are members of the TGFβ superfamily and bind to two types of serine-threonine kinase receptors (BMPRII/ActRIIA and ALK3/ALK6). Signal transduction occurs through the SMAD 1/5/8 pathway [12]. The proteins are highly expressed in the theca cells of antral follicles, while receptors are found in the follicular epithelium and oocytes. In addition to follicular activation, known functions of BMP4 and BMP7 include promoting ovulation, aromatase synthesis by granulosa cells, promoting estrogen secretion, and inhibiting progesterone in antral follicles [13].
KGF, PDGF, and LIF upregulate Kitl in granulosa cells. In primordial follicles, FGF7 and PDGF are expressed in oocytes and surrounding stromal cells (expression patterns differ between humans and rodents [14]), and LIF is expressed in pregranulosa and somatic cells [10]. Despite the participation of the presented factors in the activation of primordial follicles, their critical role in ensuring reproductive function must be clarified. For example, KGF-deficient mice (FGF7 –/–) have no birth defects. LIF knockout (LIF –/–) causes implantation failure, but overall normal folliculogenesis and ovulation are observed [10]. The ability of many molecules to influence Kitl expression is likely a consequence of evolutionarily established “redundant” signal transduction pathways and provides compensation for some factors when others are deficient.
Transcription factors of multiple signaling pathways that regulate the first step of folliculogenesis also include Foxl2, GDF9, Nobox, SOHLH1, AMH and neurotrophins.
In addition to FOXO3, another factor of the fork head family that plays a key role in the transition of primordial follicles to the growing pool is Foxl2 (For khead box L2). In mice, the protein is expressed in pregranulosa cells, and Foxl2 expression is reduced in preantral follicles. The factor is critical in the transition of the follicular epithelium from squamous to cubic at the beginning of follicular growth. In Foxl2-deficient animals (Foxl2 –/–), primordial follicles are formed, but they do not develop into secondary follicles as a result of a defect in granulosa cell differentiation. A large proportion of oocytes are activated prematurely, as evidenced by the expression of GDF9 (growth/differentiation factor 9). The proliferation block slows down the growth of oocytes and leads to follicular atresia [15].
GDF9 is one of the leading growth factors of oocyte origin, providing the functions of somatic cells of the environment [16]. The protein is directly regulated by Nobox. GDF9 is expressed in oocytes of growing follicles. Treatment of immature rats with recombinant GDF9 results in increased rates of follicular activation and follicular growth. Factor knockout in mice (GDF9 –/–) causes oocyte growth due to Kitl upregulation (increased Kitl/Kit signaling, increased Kit expression). At the same time, the level of inhibin α sharply increases, leading to a decrease in the proliferation of folliculocytes and the absence of conditions for the formation of the thecal membrane. As a result, GDF9 –/– follicles do not form beyond the primary stage. The results suggest that GDF9 plays a role in follicular cell recruitment and at the same time limits oocyte growth [10]. Expression patterns, which are detected mainly in oocytes of growing follicles, demonstrate the role of the factor in the processes of further follicular development rather than in the recruitment of primordial follicles.
Nobox (newborn oocyte-specific homeobox protein), Sohlh1 and Sohlh2 (spermatogenesis- and oogenesis-specific helix-loop-helix proteins 1 and 2) are critical transcription factors for the transition of follicles from the primordial to the growing pool. All three proteins are found in germ cell clusters, primordial and primary follicles. Sohlh1 and Sohlh2 disappear quickly once oocytes reach the secondary stage of development, Nobox is detected in all stages of folliculogenesis. Mice lacking any transcription factor are sterile. Despite the fact that the ovaries from newborn knockout Nobox –/– or Sohlh1 –/– individuals contain a similar number of cell clusters of germ cells and primordial follicles as controls, the growth of follicles beyond the primordial stage is impaired. Sohlh2-deficient animals (Sohlh2 –/–) have a similar Nobox –/– or Sohlh1 –/– phenotype, but sometimes form follicles with several layers of granulosa [3, 10]. In contrast to the molecular biological changes in Nobox –/– ovaries, in newborn Sohlh1 –/– or Sohlh2 –/– mice there is downregulation of the Kit receptor, which emphasizes the redundancy of signaling pathways for the formation of primordial follicles in the prenatal period and their activation in the postnatal period .
AMH (anti-Mullerian hormone) and its receptor AMnRII in humans and rodents are expressed in folliculocytes of primary and preantral follicles. The role of AMH in the primordial follicle was investigated in Amh –/–. Although female mice lacking AMH are fertile, there is premature depletion of primordial follicles (AMH inhibits their growth). When culturing neonatal mouse ovaries (as well as human ovarian tissue) in the presence of recombinant AMH, the number of growing follicles is reduced on average by 2 times. Currently, AMH is the only exogenous negative regulatory factor of follicular activation [17]. In humans, it is difficult to establish a direct relationship between the concentration of AMH and the pool of primordial follicles (in mice there is a strong correlation between these parameters). However, in women, AMH is positively correlated with the number of antral follicles [3, 10].
NTs (neurotrophins) are soluble growth factors whose functions extend beyond the nervous system and include the regulation of early folliculogenesis. At least 4 of the 5 NTs, as well as their receptors, are expressed in the ovary: NGF (nerve growth factor), BDNF (brain-derived neurotrophic factor), NTF3 and NTF5 (neurotrophins 3 and 5). NTs are expressed in somatic cells and oocytes of follicles before they begin to grow. In the ovaries of NGF-deficient animals (NGF –/–), mainly primordial follicles are found; the population of primary follicles is small. The gonadal phenotype is similar to Foxl2 –/–, and NGF signaling is also important in differentiating folliculocytes from squamous to cuboidal forms upon follicle activation. Although mice lacking the common p75 NGF receptor are able to reproduce without defects in follicular formation, deficiency of NTRK1 (high-affinity receptor tyrosine kinase NGF) causes perinatal lethality, and the ovarian effects of NTRK1 remain unknown [3, 10].
Basal follicular growth (follicle growth from initiation to the small antral stage - 2 mm in diameter). At the early stages of development, the primary follicle is represented by cubic folliculocytes, forming a single layer around the oocyte (the diameter of which is ~ 25 μm). During preantral folliculogenesis, the structure of the follicle changes, mainly due to the proliferation of follicular cells, which already form several layers, as well as the acquisition of theca. The shape of the folliculocytes becomes cylindrical, the size of the oocytes continues to increase. The main events that occur in the primordial follicle include FSH (follicle stimulating hormone) receptor expression, oocyte growth and differentiation. FSH receptors in the follicular epithelium of primary follicles are detected shortly after the initiation of follicular growth. The key stimulators of FSH receptor expression are FSH itself, activin, cAMP (cyclic adenosine monophosphate) and TGF. Although the recruitment and initial stages of follicular growth are independent of gonadotropins, FSH is required for the development of the primary follicle to the preantral stage. A. Hsueh et al. [18] propose to use two terms to designate the preantral and antral stages of follicular growth - “FSH-sensitive” and “FSH-dependent” periods. As a result of reactivation of the oocyte genome in the period from the primordial to preantral stages, the size of the germ cell increases 5 times, the surrounding extracellular matrix forms the zona pellucida
) [1].
Central regulators of zona pellucida
(zp1, zp2, zp3) are the transcription molecules FIGLA (germ lineage factor α), Nobox and growth factors GDF9, BMP15 [1].
Zp-deficient mice are infertile or subfertile because there is no growth or further development of follicles. FIGLA is required for the formation of primordial follicles prenatally [19]. When the transcription factor Figα (Figla –/–) is removed, the number of clusters of germ cells is similar to the control, but in the postnatal period there are no primordial follicles. Figla –/– ovaries demonstrate a decrease in the activity of 165 genes and an increase in the activity of 38 genes, most of which encode transcription factors for essential nucleic acid functions [10].
The greatest role in the regulation of preantral folliculogenesis is assigned to oocyte-secreted factors, primarily GDF9 and BMP15 [20, 21]. Both factors are produced by oocytes and stimulate the proliferation of granulosa cells and the development of theca [10, 22]. Dysregulation of GDF9 has been noted in polycystic ovary syndrome, and BMP15 mutation is associated with premature ovarian failure [1]. The importance of GDF9 and its regulator Nobox in basal follicular growth is presented above.
BMP15 belongs to the TGFβ superfamily [23]. The amino acid composition of the factor is more than half identical to GDF9, due to which the expression patterns of both proteins are similar. However, BMP15 is not required during preantral folliculogenesis in mice. BMP15 –/– animals are subfertile, as the number of follicles at later stages of development and corpus luteum decreases due to the loss of oocytes. At the same time, the relationship between the importance of GDF9 and BMP15 in the processes of early and late folliculogenesis in different mammalian species may vary. For example, BMP15 –/– sheep show an ovarian phenotype similar to that of mice GDF9 –/–, which demonstrates the critical role of BMP15 specifically in the processes of early folliculogenesis, the redundancy of regulatory pathways and, probably, evolutionary features. BMP15 expression is regulated by FSH via Kit signaling. In addition to their powerful mitogenic function, for folliculocytes GDF9 and BMP15 also inhibit apoptosis and premature luteinization, stimulate energy and cholesterol metabolism, and participate in the expansion of cumulus cells. At later stages of folliculogenesis in follicular epithelial cells, factors interact with morphogenetic protein II receptors and the kinase-like activin receptor, stimulate SMA- and MAD-related intracellular signaling, participating in oocyte maturation [24].
The third oocyte proliferation factor of the follicular epithelium is R-spondin2. The protein is a stem cell growth factor that acts through the Wnt signaling pathway. The involvement of R-spondin2 in preantral folliculogenesis was first demonstrated by Y. Cheng et al. [25]. Under natural conditions, administration of the R-spondin agonist stimulates the development of follicles to the preantral stage in both immature mice and adults desensitized by administration of the GnRH antagonist.
In addition to oocyte factors, a large group of protein ligands secreted by granulosa cells and acting through RTK (receptor tyrosine kinases) modulate follicular growth: IGF1, KGF, VEGF, FGF2, FGF10, activins, BMP6, AMH, PACAP, VIP, CNP, CTGF . The ovarian role of RTK-mediated signaling of some ligands is presented above, key others are discussed below. However, the physiological significance of these factors in preantral folliculogenesis is necessary, but less obvious [18].
After activation of primordial follicles, Kit/Kitl signaling persists and is also critical for oocyte growth and theca cell organization around follicles [26]. It is likely that several factors influence follicular growth by stimulating folliculocytes to increase Kitl expression [27]. Increased expression of Kitl in the follicular epithelium enhances the effect on oocytes, further demonstrating a “direct” paracrine interaction between the reproductive and somatic cells of the environment [1, 10].
The stimulatory functions of neurotrophins are also maintained during preantral folliculogenesis. The signals of NTF5 and BDNF to the oocyte through NTRK2 during the growth of primary follicles are probably redundant. Loss of Ntf5 does not change the number of preantral follicles, but combined inactivation of Ntf5 and BDNF significantly reduces their number, producing a phenotype similar to Ntrk2 –/–. The action of NTF5 and BDNF is independent of the signaling pathways associated with Gdf9 and Kitl [10].
An important event in the development of the primary follicle is the formation of intimate intercellular connections between oocytes and folliculocytes. Both cell types form numerous cytoplasmic projections and microvilli that extend onto each other to increase the contact area. Some microvilli of epithelial cells reach the nuclear membrane of the germ cell. Numerous gap junctions (intercellular channels) are represented by proteins - connexins (Cx), which ensure the diffusion of ions, metabolites and signaling molecules. Several Cx are expressed in the mammalian ovary, with at least two playing significant and distinct roles during folliculogenesis. Cx37 (GJA4) is the predominant gap protein synthesized by oocytes following follicular activation. Folliculocytes, on the contrary, mainly produce Cx43 (GJA1). Between oocytes and granulosa cells, heterotypic gap junctions are formed, consisting of both Cx, while between follicular epithelial cells, homotypic gap junctions are formed, represented only by Cx43. The significance of gap junction interactions has been demonstrated in rodents. Cx43 knockout mice (Gja1 –/–) die in the early postnatal period due to cardiac malformations. The ovaries of newborn individuals are small, which is due to a block in the initial stage of follicular development, impaired proliferation of folliculocytes and slow growth of oocytes with defects in meiotic maturation. Cx37-deficient ovaries (GJA4 –/–) also contain incompetent oocytes, and follicular growth progresses only to the late preantral stage. Structures resembling the corpus luteum are found in the gonads. Accordingly, communication through Cx37 is the main mechanism for regulating the formation of corpus luteum, and when the oocyte-folliculocyte interaction is disrupted, premature luteinization of follicles occurs [1, 10].
After the follicle acquires two layers of folliculocytes, a morphologically distinguishable layer of somatic cells develops. Mesenchymal cells found around the basement membrane produce BMP4 [28]. Expression of the factor in stromal cells is probably necessary not only for the activation of primordial follicles, but a functional marker is a condition for the differentiation of theca cells. The interstitial cells surrounding the folliculocytes (theca interna) have ultrastructural features: a large number of mitochondria with tubular cristae, a smooth endoplasmic reticulum, as well as numerous lipid vesicles corresponding to their main function; they are a source of androgens for the synthesis of estrogens in granulosa cells. The theca externa is composed of fibroblasts, smooth muscle cells and macrophages and is important during ovulation. Theca formation during preantral folliculogenesis is gonadotropin-independent, since the progenitor cells lack LH receptors and the theca layer forms in the ovaries of FSH-deficient mice. Further development of the thecal membranes is accompanied by the new formation of numerous small blood vessels. Consequently, nutrients and gonadotropins circulate through the bloodstream around the developing follicle. Before the follicle enters the antral stage of growth, the latter contains five histologically distinct but interacting structural units: a mature oocyte surrounded by a zona
pellucida
, about 9 layers of follicular epithelial cells and 2 layers of theca with a capillary network outside the basement membrane [1, 10].
The factors regulating thecal differentiation have not been definitively established, but are thought to be small molecules of 20–25 kDa secreted by growing follicles. Potentially promoting theca cell differentiation markers include IGF (insulin-like growth factors), Kitl and GDF9. When undifferentiated rat theca-interstitial cells were cultured in artificial medium with mRNA for LH/choriogonadotropin receptors (Lhcgr) and Cyp17a1, androgen production began. IGF1 increases the expression of Lhcgr, Cyp11a1 and 3β-hydroxysteroid dehydrogenase (Hsd3b1), and with the addition of Kitl, the mRNA of other markers is detected - Star (steroidogenic acute regulatory protein) and Cyp17a1 [1, 10].
Some effects of GDF9 on theca development are indirect [29]. Gdf9 –/– ovaries demonstrate the absence of Cyp17a1, Lhcgr and Kit markers, despite a large number of thecal cell precursors in the interstitium and high concentrations of FSH and LH. Recombinant GDF9 in mice regulates IGF1 in cultured granulosa cells. The action of the factor may be even more important in the differentiation of theca cells, since the double knockout of inhibin α and GDF9 is accompanied by the formation of a morphologically distinguishable layer of the theca interna, but devoid of most of the above markers of their activity.
In the antral gonadotropin-dependent stage of growth, LH stimulation leads to the expression of key enzymes of steroidogenesis in theca cells: CYP11A1, HSD3B1 and CYP17A1. LH also upregulates Star, which transports cholesterol to the inner mitochondrial membrane where CYP11A1 is located. Folliculocytes respond to FSH by upregulating CYP19A1 and 17b-hydroxysteroid dehydrogenase (HSD17B1). Since estradiol is essential for the later stages of folliculogenesis and ovulation, and thecal endocrinocytes express the LH receptor long before the formation of the follicular cavity, it is necessary to suppress excess androgen biosynthesis in preantral and small antral follicles. The follicular epithelium secretes activins that modulate the effects of LH on theca cells. Kitl of folliculocytes also regulates theca factors that have an autocrine inhibitory effect on the production of androstenedione: TGFβ, TGFα, FGF7 and HGF (hepatocyte growth factor) [1, 10].
The end of preantral folliculogenesis is accompanied by such changes in oocytes that spontaneous resumption of meiosis is possible when it is removed from the follicular environment. However, it is known that meiosis is rarely activated in oocytes during folliculogenesis. A decisive role in regulating the meiotic competence of oocytes is played by cAMP, which suppresses the resumption of meiosis [1]. The action of FSH is mediated primarily by cAMP signaling within the oocyte itself. In addition to FSH, CNP (C-type natriuretic peptide) is a paracrine follicle-stimulating factor, including at the preantral stage. CNP activates NPR2 (receptor, guanylate cyclase B), which provides signaling to the second messenger cGMP (cyclic guanosine monophosphate). There is evidence that it is cGMP (and not cAMP) that passes through gap junctions from folliculocytes into the oocyte, where it inhibits the function of PDE3A (phosphodiesterase 3A), preventing PDE3A-mediated cleavage of cAMP and thus inhibiting meiosis [18].
It has been known for almost 100 years that ovarian damage can promote follicular growth [18], but only now has a convincing scientific basis for this position emerged. The pioneers of creating a new understanding of early folliculogenesis are scientists at Stanford University led by A. Hsueh. Researchers have shown that the Hippo signaling pathway limits and CNN growth factors stimulate preantral follicular growth [18].
Hippo is a leading evolutionary signaling pathway that controls organ size in all multicellular organisms, inhibits cell proliferation and stimulates apoptosis (determines the further development of stem/progenitor cells) [30]. The signaling consists of several negative growth regulators acting on the serine/threonine kinase cascade, ultimately phosphorylating and inactivating the key transcriptional coactivators YAP and TAZ (effectors of the Hippo suppressor pathway with proliferative and oncogenic activities). When Hippo signals are activated, coactivators are sequestered in the cytoplasm by various proteins (14−3-3 proteins) that promote their degradation. When Hippo signaling is disrupted, nuclear YAP/TAZ interacts with the transcription factor TEAD of follicular epithelial cells and increases the expression of growth factors (CCN) and inhibitors of apoptosis (BIRC). The Hippo signaling pathway is regulated by the physical and mechanical microenvironment of cells. Mechanical signals from the extracellular matrix, sites of cell adhesion, and the state of the actomyosin cytoskeleton come together in the Hippo pathway and influence the further fate of cells [31]. Damage to the gonads (extracellular matrix) polymerizes G-actin to F-actin, disrupts Hippo signaling - activates YAP/TAZ.
After activation and exit from the dormant state, local Hippo signals determine different growth trajectories of follicles, associated with different life expectancies (23–90 days) [18]. Primordial follicles are located in the cortical region, the extracellular matrix of which is more rigid than that in the medullary region. Culturing mesenchymal stem cells in a hard extracellular matrix increases YAP activity, while in a soft matrix the effect of YAP is reduced [32]. One of the likely mechanisms for maintaining follicles in an inactive state (along with intra-oocyte signaling pathways) is Hippo mechanotransduction, since it is known that as follicles grow, they move away from the surface of the ovaries, where YAZ activity increases. Larger follicles increase Hippo signaling in nearby smaller follicles, inhibiting their growth. During each ovulation, pronounced structural changes caused by follicular rupture can disrupt local Hippo signaling and induce actin polymerization near the ovarian surface. Monthly disruption of signaling, ultimately leading to “overproliferation” of the superficial coelomic epithelium, may contribute to the formation of ovarian cancer. Defects in the genes of the Hippo signaling pathway are associated not only with oncogenesis (LATS ½), mechanotransduction also occurs in primary ovarian failure (DIAPH2, BIRC1), polycystic ovary syndrome (LATS ½) and decreased ovarian reserve in infertility (DIAPH2, DIAPH3, CCN2) [ 18].
Thus, the regulation of early follicular growth has a complex mechanism, including several signaling pathways, intra- and extra-oocyte factors. Despite the research, the mechanisms of initiation of basal follicular growth, as well as the factors regulating thecal differentiation, have not yet been determined. However, there is no doubt that the presented regulatory mechanisms are the basis for maintaining the ovarian reserve. A detailed study of the signaling pathways regulating follicular growth will provide new opportunities for the prevention and treatment of ovarian dysfunction, which is the main goal of reproductive biology and medicine.
The authors declare no conflict of interest.
What violations can be detected?
Ultrasound will help detect the following pathological conditions:
- stopping the growth of the follicle, its reverse development and failure to ovulate;
- the follicle does not rupture, and therefore the egg does not release;
- follicular cyst - a condition in which fluid can accumulate in the follicle;
- complete lack of follicle development, which may be caused by hormonal imbalance.
| Ultrasound of the female genital area | Price, rub.) |
| Folliculogenesis with CDC (1 examination) | 350 |
| Transvaginal ultrasound of the uterus and appendages with color doppler | 700 |
| Transabdominal ultrasound of the uterus and appendages with color circulation | 450 |
| Transvaginal ultrasound to determine pregnancy | 700 |
| Cervicometry | 350 |
| Ultrasound of the urethra | 550 |
Studying folliculogenesis is an important step in preparing for natural conception and pregnancy.
To sign up for diagnostics at the Altai Medical Center clinic, you can use the online form on the website or call: (3852)222-250 and (3852)222-250. Make an appointment