Hypoplastic anemia
– a blood disease characterized by a decrease in the hematopoietic function of the bone marrow.
Hypoplastic and aplastic anemia are associated with impaired hematopoietic function of the bone marrow, but there are differences between them.
Symptoms of hypoplastic anemia
There are several types of the disease:
— Subacute hypoplastic anemia;
— Chronic hypoplastic anemia;
— Chronic hypoplastic anemia with a hemolytic component;
— Partial hypoplastic anemia.
Chronic hypoplastic anemia is characterized by a long course with periodic exacerbations. This type of disease causes the patient weakness, increased fatigue, shortness of breath, and rapid heartbeat.
Patients with hypoplastic anemia with a hemolytic component may develop a yellow tint to pale skin.
Diagnosis of hypoplastic anemia
The diagnosis is made based on the patient's life history and examination, symptoms and laboratory tests.
A blood test reveals a decrease in the number of reticulocytes, platelets, and red blood cells. In a biochemical blood test, an increased amount of serum iron is observed.
Bone marrow examination shows a decrease in erythrocyte and granulocyte cells and lymphocytes. Sometimes there is devastation of the bone marrow and an increase in the amount of iron in it.
Aplastic anemia
Aplastic anemia is a severe blood disease in which suppression of all bone marrow sprouts develops. The causes of the disease are various - from genetic predisposition to the harmful effects of ionizing radiation and various chemical compounds. Clinically, the disease manifests itself as anemic, thrombocytopenic syndrome, as well as severe infectious complications. The diagnosis is made based on the clinical picture, blood tests and bone marrow puncture.
What is aplastic anemia?
Aplastic anemia is a pathological condition of the body in which the number of all three types of blood cells (erythrocytes, leukocytes and platelets) decreases due to a slowdown or complete cessation of their formation in the bone marrow. By origin, aplastic anemia is divided into:
- congenital;
- acquired.
In most cases, inhibition of all three germs of hematopoiesis is observed, however, it has been clinically observed that in different phases of the disease a more pronounced inhibition of one of the germs may be observed
Based on the predominant damage to bone marrow sprouts, the following are distinguished:
- inhibition of one hematopoietic lineage (erythrocyte, leukocyte or platelet);
- inhibition of two hematopoietic germs;
- inhibition of three hematopoietic germs.
Causes of aplastic anemia
The causes of aplastic anemia differ between congenital and acquired anemia.
The following causes of acquired aplastic anemia are distinguished:
- ionizing radiation;
- medications (decaris, analgin, chloramphenicol, tetracycline, butadione, etc.);
- chemical compounds (pesticides, benzene);
- diseases (viral hepatitis A, B and C, Epstein-Barr virus, cytomegalovirus, herpes virus, HIV, parvovirus B19, etc.).
- hormonal disorders of the ovaries, thyroid gland and thymus gland.
Some harmful agents directly affect the bone marrow (ionizing radiation, chemicals and drugs). Others act indirectly through autoimmune mechanisms (viral hepatitis B).
Diagnosis of aplastic anemia
The clinical picture of the disease can largely guide the doctor in the direction of anemia, but the diagnosis must be confirmed or refuted using laboratory tests and paraclinical studies. The most valuable additional studies are:
- complete blood count (CBC);
- biochemical blood test (BAC);
- sternal puncture;
- trepanobiopsy.
General blood analysis
Data from a general blood test for aplastic anemia indicate pancytopenia (a decrease in the number of all three types of bone marrow cells). A decrease in the number of leukocytes is observed mainly due to a decrease in granulocytes (neutrophils, eosinophils and basophils). Thus, the percentage of lymphocytes and monocytes in the leukocyte formula increases relatively. At various stages of the disease, inflammatory signs to one degree or another can be detected. Indicative indicators of UAC for aplastic anemia are:
Hemoglobin (Hb) – less than 110 g/l (normal 120 – 160 g/l). Reduction due to a decrease in the number of red blood cells.
Red blood cells – 0.7 – 2.5 x 1012\l (normal 3.7 x 1012\l). Decrease in the number of mature red blood cells.
Reticulocytes - less than 0.2% (normal 0.3 - 2.0%). Decrease in the number of young forms of red blood cells.
The color index is 0.85 – 1.05 (the norm is 0.85 – 1.05) indicates the normochromic nature of anemia (the hemoglobin content in the erythrocyte is within the normal range).
Hematocrit (Ht) – less than 30 (normal 35 – 42 in women and 40 – 46 in men). The ratio of the cellular composition of blood to its liquid part. There is a clear decrease in the proportion of cells in the peripheral blood.
Platelets – less than 35 ppm or 100 x 109\l. Decreased platelet count.
Leukocytes – 0.5 – 2.5 x 109\l (normal 4 – 9 x 109\l). Severe leukopenia due to a decrease in the number of granulocytes (neutrophils, eosinophils and basophils).
Band neutrophils – 0 – 2% (normal is less than 6%). Decreased production of young forms of leukocytes.
Segmented neutrophils – 0 – 40% (normal 47 – 72%). Decrease in the number of mature forms of neutrophils.
Myelocytes – 0 – 2% (normally absent). In conditions of granulocytopenia and bacterial infection, a more pronounced than usual shift in the leukocyte formula to the left is observed with the appearance of leukopoiesis precursor cells.
Eosinophils – 0 – 1% (normal 1 – 5%). Decrease in the number of eosinophils.
Basophils – 0% (normal 0 – 1%). Single or complete absence of basophils.
Lymphocytes - more than 40% (normal 19 - 37%). The number of lymphocytes remains normal. Due to a decrease in the granulocyte fraction, relative lymphocytosis is observed (an increase in the proportion of lymphocytes in the blood). Extremely pronounced lymphocytosis can be observed with the accumulation of viral infections.
Monocytes – more than 8% (normal 6 – 8%). The number of monocytes is unchanged and within normal limits. Monocytosis (increased proportion of monocytes in the blood) is explained by a decrease in the percentage of granulocytes in the leukocyte formula.
The erythrocyte sedimentation rate is more than 15–20 mm/hour (the norm is up to 10 mm/hour in men and up to 15 mm/hour in women). This indicator reflects the severity of the inflammatory response in the body.
Anisocytosis is the presence of red blood cells of various sizes in the blood.
Poikilocytosis is the presence of red blood cells of various shapes in the blood.
Blood chemistry
Some types of biochemical blood tests can focus the doctor’s attention on abnormalities in the body that indirectly fit into the three anemic syndromes listed above. Approximate indicators of BAC for aplastic anemia are:
Serum iron is more than 30 µmol/l (normal 9 – 30 µmol/l). Increased serum iron levels due to frequent blood transfusions. High risk of developing hemochromatosis.
Erythropoietin more than 30 IU/l (normal 8 – 30 IU/l in women and 9 – 28 IU/l in men). The increase in erythropoietin occurs for two reasons. Firstly, it is not consumed by the cells of the erythrocyte lineage. Secondly, its synthesis increases compensatoryly in response to anemia.
C-reactive protein – more than 10 – 15 mg/l (normal 0 – 5 mg/l). It is detected during an inflammatory reaction against a background of weakened immunity.
Thymol test - more than 4 (norm 0 - 4). Detects signs of inflammation in weakened immune systems.
Sternal puncture
This type of study is used to visualize bone marrow cells and their percentage. With aplastic anemia, the myelogram will be scanty, the number of cellular elements is significantly reduced. Cambial cells of the erythrocyte and leukocyte series are single or absent. Megakaryoblasts are absent. In rare cases, during puncture it happens to encounter grouped foci of increased cell proliferation as a compensatory reaction of healthy bone marrow to anemia. Such a myelogram may be misleading because it will indicate the absence of aplastic anemia and will therefore be false negative.
Trephine biopsy
Trephine biopsy is a method of removing part of the bone marrow from the patient's ilium wing. The advantage of this procedure over sternal puncture is the possibility of collecting a larger amount of material while maintaining its structure. A larger amount of material reduces the likelihood of a false negative result of aplastic anemia, and studying the structure of the bone marrow allows, in addition to a cytological examination (myelogram), to also conduct a histological examination. Using a blood test and trepanobiopsy results, it is possible to determine the severity of aplastic anemia.
- Aplastic anemia of moderate severity is determined by the following indicators:
granulocytes less than 2.0 x 109\l;
platelets less than 100 x 109\l;
reticulocytes less than 2 – 3%;
bone marrow hypoplasia on trephine biopsy.
- Severe aplastic anemia is determined by the following indicators:
granulocytes less than 0.5 x 109\l;
platelets less than 20 x 109\l;
reticulocytes less than 1%;
bone marrow aplasia on trephine biopsy.
- Extremely severe aplastic anemia is determined by the following indicators:
granulocytes less than 0.2 x 109\l;
platelets are single or absent;
reticulocytes are single or absent;
bone marrow aplasia on trephine biopsy.
Prognosis for aplastic anemia
The prognosis for aplastic anemia largely depends on the timing of detection of the disease. With early detection, there is the possibility of more active intervention in the course of the disease. If detected later, the chances of cure decrease. Congenital Fanconi aplastic anemia is in most cases extremely difficult to treat, since the bone marrow has never been healthy and, accordingly, is very difficult to recover. The presence of congenital developmental anomalies greatly limits the indications for bone marrow transplantation in such patients. In most cases, patients die in childhood from developmental abnormalities or infectious complications. Acquired aplastic anemia has a more favorable prognosis, since in some cases it is reversible after the cessation of the action of the damaging factor on the bone marrow.
Laboratory diagnostics doctor
Novopolotsk city hospital
Kostyuk K.S.
Treatment of hypoplastic anemia
Hypoplastic anemia cannot be completely cured, but therapeutic therapy can increase the life expectancy of patients.
The underlying disease that caused hypoplastic anemia is treated, and the factors that provoked it are eliminated.
Patients receive transfusions of red blood cells and platelets. A course of B vitamins, ascorbic acid, rutin, and calcium chloride is also prescribed. It is possible to carry out hormonal therapy with corticosteroids, which prolong the life of red blood cells and reduce the body's response to blood transfusion.
Surgical treatment involves removal of the spleen, thereby reducing the depressive effect on the bone marrow and the level of destruction of red blood cells. In some cases, a bone marrow transplant is used, but this requires careful selection of a donor.
The prognosis of hypoplastic anemia is unfavorable in the acute and subacute course of the disease. In other cases, therapeutic therapy reduces the percentage of deaths and increases the quality of life of patients.
Anemia, pathology of hemostasis, oncohematology
Materials are presented from the RUDN textbook
Anemia. Clinic, diagnosis and treatment / Stuklov N.I., Alpidovsky V.K., Ogurtsov P.P. – M.: Medical Information Agency LLC, 2013. – 264 p.
Copying and reproducing materials without indicating the authors is prohibited and is punishable by law.
Aplastic anemia (AA) is a disease resulting from the disappearance or sharp decrease in pluripotent stem cells in the bone marrow, the number of which decreases to 1% or below, which leads to devastation or aplasia of the bone marrow. Morphologically, this is manifested by pancytopenia in peripheral blood tests, the absence of hematopoietic cells in bone marrow puncture. In the blood, pancytopenia is characterized by leukopenia due to neutropenia with relative lymphocytosis, the absence of reticulocytes. If such changes are detected, it is necessary to exclude all possible causes of aplasia (viral infections, malignant diseases, blood diseases (lymphoproliferative diseases), systemic collagenoses, contact with chemicals, radiation), and in adults, perform a trephine biopsy for histological examination of the bone marrow.
AA was first described by Ehrlich in 1888. The incidence of AA is 2–4 cases per 1 million population in the USA and Europe, while in some East Asian countries AA is much more common. Thus, in Thailand and Japan, the incidence of AA is 11–14 cases per 1 million population.
AA is most often detected in two age groups: 20–25 years and over 60 years. There is no statistically significant difference in incidence between men and women.
Etiology and pathogenesis
AA can be congenital, but more often it is acquired. Congenital, genetically determined AA were first described by Fanconi in 1927.
Congenital AA is clinically manifested by bone marrow failure in the first years of a child’s life and is often combined with congenital dysplasias such as skin dyspigmentation, hypoplasia of the kidneys and spleen, absence or hypoplasia of the radius, microcephaly, congenital heart defects and mental or sexual underdevelopment. Karyological studies in this variant of AA often reveal various chromosomal abnormalities. Among children with Fanconi anemia, there is also a high incidence of acute leukemia and other neoplasias.
In secondary AA, bone marrow failure is caused either by direct toxic effects of radiation or chemical compounds on stem cells, or by an aberrant response caused by viral infections (hemophagocytic syndrome). The most common diseases that provoke the development of AA are malignant diseases, blood diseases (lymphoproliferative diseases), and systemic collagenoses. Enlargement of peripheral lymph nodes, liver and spleen is not typical for AA. Moreover, the discovery of an enlarged spleen at an early stage of the disease casts doubt on the diagnosis of AA and argues in favor of hepatitis-associated aplasia. However, a long course of the disease may be accompanied by an enlargement of the liver and spleen due to post-transfusion hemosiderosis. In cases with an identified cause of AA, this condition should be considered as secondary hematopoietic aplasia.
True (idiopathic acquired) AA is a condition associated with the death of unchanged stem cells. In idiopathic forms, in which the cause of bone marrow aplasia is unclear, T-cell-mediated destruction of pluripotent hematopoietic stem cells of the bone marrow is assumed. In patients with the idiopathic form of AA, an increased number of activated cytotoxic T-lymphocytes, increased production of γ-interferon and tumor necrosis factor, which cause the death of their own bone marrow stem cells, were found in the blood. Moreover, the reason for the autosensitization of T lymphocytes against their own stem cells remains unclear.
Classification of aplastic anemia.
1. Idiopathic aplastic anemia
- congenital (Fanconi anemia)
- acquired
2. Secondary aplastic anemia caused by:
- medications (chloramphinecol, non-steroidal anti-inflammatory drugs, anticonvulsants, cytotoxic drugs)
- due to exposure to ionizing radiation
— chemical influences (benzene and its derivatives, pesticides, paints and varnishes)
— viral infections (Epstein-Barr, hepatitis, parvavirus, cytomegalovirus, HIV)
— autoimmune diseases (SLE, eosinophilic fasciitis, hyperimmunoglobulinemia)
- other reasons (pregnancy, thymoma)
AA Clinic
All clinical manifestations of AA are a consequence of bone marrow failure, their intensity depends on the severity and rate of progression of pancytopenia. The course of AA can be acute, subacute and chronic.
Patients with AA usually have an anemic syndrome in combination with hemorrhages on the skin, mucous membranes and infectious complications. The dominant symptoms of AA differ depending on the timing of the disease.
It is known that red blood cells circulate in the peripheral blood for 3–4 months, so symptoms of anemia can develop only with prolonged suppression of hematopoiesis for more than 1–2 months. As a result of a prolonged lack of red blood cell production, a gradual decrease in hemoglobin concentration occurs by 25–50%, that is, to 90–70 g/l, which may cause symptoms of anemia.
On the contrary, with the rapid death of the overwhelming number of stem cells, within a week hematopoiesis is depleted, the production of platelets and leukocytes stops, and the number of circulating red blood cells remains normal. Then, within a week, the number of platelets decreases, which leads to the appearance of hemorrhagic syndrome, against the background of a complete absence of anemic complaints. Before bleeding occurs, the body compensates for the lack of platelets using existing platelets, which are normally viable for an average of 10–11 days. Moreover, the decrease in platelets in the circulation occurs several days earlier than the appearance of hemorrhages, since the main number of them is represented by the parietal pool, which makes up the bulk of peripheral blood platelets and is consumed more slowly. Hemorrhagic syndrome with thrombocytopenia is associated with inadequate trophism and damage to the endothelium, increased fragility of small vessels and is manifested by bleeding of the mucous membranes (nasal, uterine bleeding), petechial rashes on the skin. Cutaneous hemorrhagic syndrome on the upper half of the body, especially on the face, is considered a life-threatening condition, which sharply increases the risk of bleeding in the brain.
As for leukocytes, especially neutrophils, their number decreases most intensively. The lifespan of granulocytes is no more than a week, and they circulate in the peripheral blood for several days, then enter the surrounding tissues, where they perform the main phagocytic function. Therefore, even against the background of a complete absence of neutrophils in the peripheral blood (agranulocytosis), infectious complications do not occur immediately, but, as a rule, after 5–7 days, and have their own characteristics. These patients often have a high fever in the absence of a source of infection. Thus, with shortness of breath, it is not possible to hear wheezing; with abdominal pain, the symptoms of peritoneal irritation are extremely blurred. To establish the diagnosis of pneumonia and peritonitis in this case, instrumental research methods (x-ray and ultrasound) are extremely important, with the use of which one can see the infiltration of lung tissue, the appearance of fluid levels in the intestines with atony, thickening of the intestinal wall and others. In patients with agranulocytosis, when fever appears, the risk of developing septic shock is extremely high due to the lack of barrier function of leukocytes. Such patients, when fever develops, necessarily need a blood test for sterility. Moreover, the diagnosis of sepsis in agranulocytosis can be established even contrary to the classical definition of sepsis, that is, without the presence of a “primary focus.” The most common cause of sepsis in agranulocytosis is “normal” intestinal flora (saprophytes or opportunistic bacteria).
In the acute course of AA, there is a rapidly progressing hemorrhagic syndrome caused by deep thrombocytopenia, and severe infectious complications due to the almost complete absence of granulocytes. Patients with a similar course of AA require emergency hospitalization in the hematology department and antibacterial, replacement, often intensive therapy, and specialized treatment. Without adequate care, such patients usually die within a few days or weeks of the first signs of the disease appearing.
In the moderate (subacute) course of AA, weakness and increased fatigue remain the patient’s main complaints for a long time; then, due to thrombocytopenia, symptoms of hemorrhagic diathesis may appear.
For secondary AA, which can clinically manifest itself weeks and even months after contact with the etiological factor, a chronic course is more typical. With secondary AA, more often than with the idiopathic form, remission of the disease develops and complete recovery may occur after the action of the etiological factor ceases.
Laboratory data
The picture of peripheral blood in 90% of patients with AA is characterized by pancytopenia: anemia, leukocytopenia and thrombocytopenia.
Anemia is normochromic in nature. The number of reticulocytes is reduced to 0 - 0.3%.
Leukopenia is caused by granulocytopenia, and the content of lymphocytes is usually not changed, which creates the impression of lymphocytosis, which is relative in AA. The severity of leukopenia largely determines the severity of the disease: the number of leukocytes <0.5 x 109/l is observed in patients with severe AA, the number of leukocytes <0.2 x 109/l is observed in patients with super-severe AA.
Thrombocytopenia is detected already in the early stages of the disease and is the most persistent hematological symptom.
The bone marrow aspirate is in the vast majority of cases very poor, i.e. contains a small number of hematopoietic cells, most of which are lymphocytes. However, since the process of bone marrow aplasia occurs unevenly and individual foci of normal hematopoiesis are preserved, it is possible to obtain active bone marrow with an almost normal cellular composition (with pancytopenia in peripheral blood analysis!). All this allows us to conclude that the myelogram in AA does not have a decisive diagnostic value.
The key method for diagnosing AA in adults is histological examination of the bone marrow. To conduct this study, a trepanobiopsy is performed, the results of which in AA indicate a total predominance of adipose tissue over active bone marrow.
Biochemical blood tests in most patients with AA reveal high levels of serum iron, increased levels of LDH and transaminases, and increased levels of erythropoietin.
The diagnosis of AA is based on a combination of peripheral blood pancytopenia, a decrease in bone marrow cellularity during sternal puncture and the detection of fatty bone marrow during histological examination, while other causes of hematopoietic aplasia are excluded.
Criteria for pancytopenia: Hb < 110 g/l, granulocytes < 2.0 x109/l, platelets < 100 x109/l.
The classification of AA according to severity is shown in the table.
Classification of AA by severity
| Form of the disease | Criteria |
| Mild AA | — the patient does not meet the criteria for severe and super-severe forms; — granulocytes > 0.5 x 109/l. |
| Heavy AA | — granulocytes <0.5 x 109/l; — platelets < 20 x 109/l. |
| Super heavy AA | — granulocytes <0.2 x 109/l. |
As can be seen from the table, the severity of AA is not determined by the hemoglobin concentration; it only takes into account severe thrombocytopenia, and the main factor determining the severity of the patient’s condition is the level of neutrophils in the peripheral blood. This is due to the existing ability to replace erythrocyte and platelet function by transfusion of erythrocyte and platelet mass obtained from donors, and the lack of methods for correcting neutropenia.
Differential diagnosis
AA sometimes has to be differentiated from diseases that may be accompanied by pancytopenia:
- acute leukemia;
- megaloblastic anemia;
- idiopathic myelofibrosis;
- hypersplenism;
- myelodysplastic syndrome (MDS).
In acute leukemia with hypoplasia of hematopoiesis, in contrast to AA, accumulations of blast cells are found in the bone marrow against the background of bone marrow hypoplasia, and in megaloblastic anemia - megaloblastic hematopoiesis.
In patients with idiopathic myelofibrosis and hypersplenism, severe splenomegaly is almost always present, which is always absent in AA. In addition, trepanobiopsy reveals myelofibrosis in the first case, and bone marrow hyperplasia in the second.
The combination of pancytopenia and bone marrow hypoplasia can be observed in 10% of patients with myelodysplastic syndrome. However, with MDS, along with hypoplasia of hematopoiesis, its dysplastic features are present in the form of megaloblastoidity, the presence of binuclear erythroblasts, Havell-Jolly bodies, sideroblasts (erythrocytes containing iron granules), sometimes an increased content of blast cells, as well as the presence of Pelger anomaly of granulocytes ( violation of nuclear segmentation) and microforms of megakaryocytes. In addition, in MDS, as a rule, chromosomal abnormalities are found that are absent in AA.
Treatment
The only treatment method for patients with AA that provides high survival rates (78–90%) and even complete recovery of patients is allogeneic bone marrow transplantation (ABMT).
ATKM from an HLA-matched donor is considered the method of choice in patients with AA if they meet the following criteria:
1. presence of an HLA-matched related bone marrow donor;
2. severe or super-severe form of AA;
3. the patient’s age is not older than 40 years;
4. short blood transfusion history.
As a rule, only siblings are used as related donors.
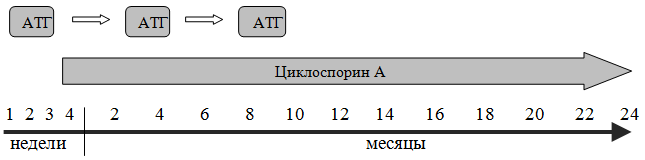
The standard mild regimen for preparing a patient (conditioning) for bone marrow transplantation includes the use of cyclophosphamide at a dose of 50 mg/kg from days 5 to 2, antilymphocyte globulin (ALG) from days 5 to 3, and methylprednisolone from days 5 to 3 . The main purpose of this conditioning is immunosuppression aimed at preventing transplant rejection, which with this regimen is reduced from 30% to 5%.
As a post-transplant immunosuppression aimed at suppressing graft-versus-host disease (GVHD), which is observed both in acute and chronic form (more often), cyclosporine A is used at a dose of 5 mg/kg per day for 12 months in combination with short courses of methotrexate 15 mg/m2 on days +1, +3, +6, +11.
The same regimen of conditioning and GVHD prevention is used when using bone marrow from HLA-matched unrelated donors. However, bone marrow transplantation from unrelated donors produces significantly worse results: only 29% of AA patients were alive 2 years after unrelated donor transplantation.
Previous blood transfusions are important in the effectiveness of bone marrow transplantation: in patients who did not receive blood transfusions, complete remission is achieved in 80% of cases with a five-year survival rate of about 70%, while in patients with sensitizing blood transfusion therapy, complete remission is achieved only in 50% of cases.
Experience in the treatment of AA with the introduction of hematopoietic cells has revealed the advantage of bone marrow transplantation over the use of mobilized stem cells obtained from the peripheral blood of a donor.
The number of mononuclear cells during bone marrow infusion should be at least 3.0x109/kg with a minimum number of CD 34+ stem cells of 2.6x106/kg.
Based on the assumption of the role of the suppressive effect of one’s own sensitized T-lymphocytes on bone marrow stem cells (with their subsequent apoptosis), the use of immunosuppressants with anti-T-lymphocyte action in the treatment of AA in patients was proposed. Like this:
- do not have HLA-compatible donors,
- patients with mild AA who depend on blood transfusions,
— patients with severe and super-severe forms of AA over 40–45 years of age.
The main therapy for AA is a regimen with a mandatory combination of pathogenetic treatment (immunosuppressive drugs and splenectomy), transfusion therapy and antimicrobial drugs.
Pathogenetic treatment
Most often, treatment begins with antilymphocyte (antithymocyte) globulin (ATG: atgam, thymoglobulin). The drugs are administered intravenously slowly over 12 hours, after a sensitivity test, the dose of the drug is 20 mg/kg/day. The total number per course is 4 introductions. To prevent serum sickness, prednisolone is used at a dose of 1 mg/kg or methylprednisolone 125–250 mg/day (before and after ATG) in combination with antihistamines (before and after ATG). From the 14th day of the course (day 1 of the course is counted from the first day of ATG administration), prednisolone must be discontinued. Cancel it a week in advance, gradually reducing the dose (daily by 1/3 - 1/2). The effect of treatment for ALH is observed in half of the patients, with complete remissions achieved in 15%.
The second immunosuppressant that is widely used in the treatment of AA is cyclosporine A (CyA), which blocks the production of interleukin-2 (IL-2), responsible for the proliferation of cytotoxic T-lymphocytes. CyA is used in a dose of 5 - 10 mg/kg per day for a duration course for at least 12 months. While taking SuA, magnesium preparations (Magne B6, Magnerot) should be prescribed, and liver (bilirubin, ALT, AST) and kidney parameters (creatinine) must be monitored. As with the use of ALG, its effectiveness is 50–60%, however, apparently, this drug does not provide sufficient immunosuppression and its withdrawal leads to relapse of the disease.
Currently, the most commonly used combination treatment regimen includes the administration of ALG for 4 days, and from the 14th day - the use of CyA for 12 months. The response rate to combination therapy is quite high and reaches 80% in severe AA with a five-year survival rate of 75%. If the combined use of ATG + CyA is ineffective, a second (after 3–6 months) and third (after 6–12 months) administration of ALG or replacement of one course of ATG with splenectomy is possible.
Treatment regimen for aplastic anemia (* - instead of ATG, splenectomy is possible)
When using ATG, serum sickness often develops. It manifests itself in 50% of patients 5–14 days after the end of drug administration with a rash, arthralgia, fever, laboratory signs of hepatitis, and increased blood pressure. In such cases, it is advisable to prescribe prednisolone at a dose of 0.5 - 1 mg/kg until the symptoms are relieved; in the absence of effect or severe symptoms of serum sickness, plasmapheresis is performed.
Transfusion therapy
In recent years, splenectomy has been performed in patients who have responded to ALG+SyA therapy, which has demonstrated its positive effect even before the introduction of immunosuppressive drugs into practice.
Maintenance (replacement) therapy, ensuring a satisfactory quality of life for patients, is of great importance in the treatment of aplastic anemia:
1. Red blood cells should be transfused when hemoglobin decreases to 80 g/l, when patients usually develop symptoms of tissue hypoxia.
2. The indication for platelet transfusion is a decrease in platelet count less than 20 x 109/l or hemorrhagic syndrome in the form of bleeding of the mucous membranes and/or in combination with rashes on the skin of the upper half of the body (during treatment with ATG, transfusions should be carried out when the platelet level is 40 - 60 109 /l).
Transfusions of blood components should be avoided in cases where the patient has HLA-compatible relatives and a bone marrow transplant is proposed. This prevents sensitization of patients and reduces the risk of transplant rejection. Long-term blood transfusion therapy leads to the development of hemosiderosis, since the erythromass packet contains 200–250 mg of iron.
Chelation therapy is used to combat post-transfusion hemochromatosis, which aggravates bone marrow failure and contributes to the development of cirrhosis of the liver and pancreas. It is started when serum ferritin increases > 1000 μg/L. The main drugs that form a complex with iron in the blood and remove it in the urine, which prevents the development of hemosiderosis, are desferal (deferoxamine) 500 mg per day intravenously (usually used against the background of blood transfusions or once a week), as well as a new one that removes excess iron is the drug exjade (deferasirox), which is conveniently administered - orally at 20 mg/kg/day.
Antimicrobial therapy
In patients with severe granulocytopenia (<0.5x109/l), CSF is prescribed, which stimulates granulocytopoiesis, but does not affect other hematopoiesis.
Patients with AA with profound neutropenia and fever are recommended to be treated with broad-spectrum antibiotics under the control of bacteriological examination of blood and urine; isolation of patients in sterile rooms is mandatory. Antibiotics must be prescribed when neutrophils drop below 0.5 x 109/L to all patients, and at normal temperatures, use prophylactic doses; when the temperature rises above 37.5 0C, use 3rd and 4th generation cephalosporins, only maximum therapeutic doses.
If there is no effect of antibiotic therapy in febrile patients, it is necessary to undergo examination for aspergillosis (galactomonan in blood serum), Pneumocystis infection (bronchoscopy with bronchoalveolar lavage - BAL), herpetic infection (PCR - in blood serum). Even with negative culture results, antifungal drugs are used: ketoconazole, fluconazole, intraconazole.
Under the influence of modern therapy, the period of extensive clinical manifestations can be replaced by a state of clinical and hematological compensation and even complete remission, in which all signs of the disease disappear. In the future, the state of clinical and hematological remission may be replaced by a relapse of the disease. Complete recovery is observed in 15–50% of patients with AA after allogeneic bone marrow transplantation.
The effectiveness of therapy is assessed after 3, 6, 9, 12, 18, 24 months from the start of therapy:
Remission (complete, partial) – complete normalization, partial normalization of blood parameters (Hb> 100 g/l, granulocytes > 1.5 x 109/l, platelets > 100 x 109/l) and absence of dependence on blood transfusions.
Clinical and hematological improvement - improvement in blood counts (Hb> 80 g/l, granulocytes > 1.0 x 109/l, platelets > 20 x 109/l) and absence or reduction of dependence on blood transfusions.
Course and prognosis
Before the use of bone marrow transplantation and immunosuppressive therapy, 25% of AA patients died within 4 months of diagnosis, and less than half lived for more than a year. Bone marrow transplantation leads to a significant improvement in the prognosis of AA and cures 80% of patients who did not receive transfusions of blood components and 60% of patients who received transfusions. Immunosuppressive therapy causes complete or partial remission in half of the patients, but some of them (15%) develop a relapse of the disease. Moreover, against the background of almost ten years of complete remission of the disease, in 40% of patients the existing stem cell defect can manifest itself in the development of paroxysmal nocturnal hemoglobinuria, myelodysplastic syndrome or acute myeloid leukemia.
When diagnosing the disease, the prognosis largely depends on the absolute number of neutrophils and platelets. An extremely poor prognosis for patients with posthepatitis aplasia.