Iron deficiency
5314 January 29
IMPORTANT!
The information in this section cannot be used for self-diagnosis and self-treatment.
In case of pain or other exacerbation of the disease, diagnostic tests should be prescribed only by the attending physician. To make a diagnosis and properly prescribe treatment, you should contact your doctor. We remind you that independent interpretation of the results is unacceptable, the information below is for reference only
Hemoglobin: indications for use, rules for preparing for the test, interpretation of results and normal indicators.
In what cases is a study prescribed?
The reason for prescribing the analysis is diagnosed bone marrow diseases, as well as general symptoms of anemia:
- pallor of the skin and mucous membranes (conjunctiva, gums, tongue);
- unjustified weakness;
- fast fatiguability;
- rapid pulse (lack of oxygen causes the heart to pump blood harder).
During a general examination, you should pay attention to specific symptoms characteristic of a particular type of anemia:
- brittle hair and nails (iron deficiency anemia);
- bright crimson tongue (B12 deficiency anemia);
- yellowness of the skin and eye sclera (hemolytic anemia)
A routine analysis of hemoglobin levels is carried out for a comprehensive assessment of the condition during pregnancy, before bone marrow transplants or interventions affecting the hematopoietic organs. Periodic monitoring requires hormonal therapy (primarily with erythropoietin preparations), as well as long-term use of iron-containing drugs.
The structure of hemoglobin and its structure
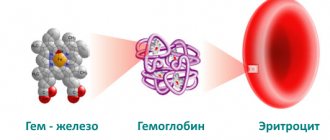
Hemoglobin has two parts in its structure - protein globin and non-protein heme; the structure of the molecule allows it to attach and release oxygen, water and carbon dioxide. Heme refers to pigments, that is, coloring substances. It gives the blood a scarlet color. There is iron inside heme. Hemoglobin contains 4 hemes, each surrounded on all sides by a chain of amino acids of the globin protein. These 4 subunits allow the binding of 4 oxygen molecules from the air in the lungs.
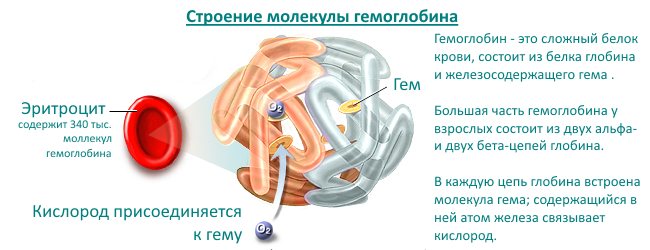
Red blood cells, consisting of 95% hemoglobin, capture oxygen molecules and carry it to the cells of the body. Hemoglobin gives off oxygen, and in return takes in water and carbon dioxide, which are also released through the lungs. The combination of hemoglobin with oxygen is called oxyhemoglobin, and with carbon dioxide - deoxyhemoglobin. All of them are normally found in red blood cells.
If hemoglobin combines with carbon monoxide, all 4 parts of the molecule are blocked, as a result of which it loses the ability to combine with oxygen. If there is nitrogen or cyanide poisoning, then iron changes from divalent (normal) to trivalent. This also disrupts the transfer of oxygen molecules, and methemoglobin is formed. A similar reaction occurs in case of poisoning with certain medications or hereditary diseases.
Kinds
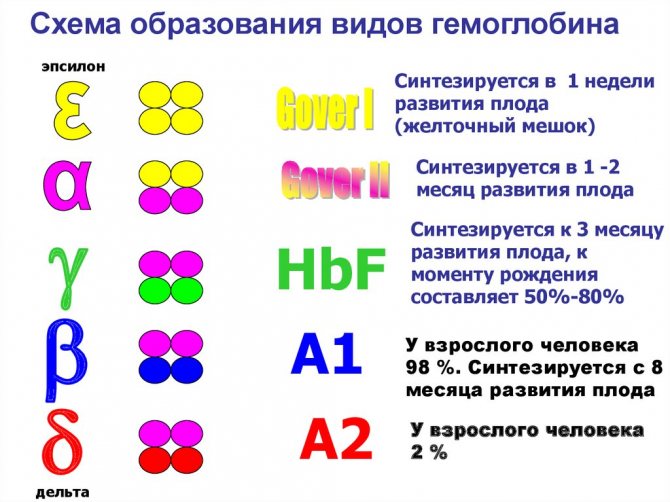
All types of hemoglobin are divided into normal (physiological) and those with a disturbed structure (pathological). To designate them, Latin letters and the abbreviation Hb (haemoglobinum) are used.
Normal forms
Normally can be found in the blood:
- mature hemoglobin HbA, it is 95-98% in an adult, and 80% in a newborn;
- fetal hemoglobin HbF (fetus means fetus) is formed from the 2nd month of pregnancy in the fetus, circulates before birth, is destroyed in the first week of life, and is characterized by a greater ability to capture oxygen;
- Fetal HbE is formed in the fetus before 2 months of intrauterine development.
Depending on what hemoglobin has attached, the following forms are distinguished:
- HbO2 – compound with oxygen (oxyhemoglobin);
- HbCO2 – hemoglobin with carbon dioxide, it is called deoxyhemoglobin;
- HbMet is methemoglobin with oxidized iron, its amount is normally allowed up to 3%.
Pathological
More than 300 forms of pathological hemoglobins are known. Most often found:
- HbS – hemoglobin in sickle cell anemia;
- HbCO – carboxyhemoglobin formed during carbon monoxide poisoning;
- HbA1C is glycosylated hemoglobin, its level increases in diabetes mellitus.
Decoding the results
To diagnose anemia, a general and/or biochemical blood test is used. They are supplemented with other diagnostic tests as needed. Particular attention is paid to the following indicators:
- hemoglobin level (normal: 115-135 g/l for women and 125-145 g/l for men);
- number of red blood cells (norms: 3.7-4.6*1012/l – for women and 4.5-5.5*1012/l – for men);
- saturation of erythrocytes with hemoglobin (norm: 0.8-1.1 units - for the general color indicator and 28-32 picograms - on average for each erythrocyte);
- size and shape of red blood cells (several dozen possible disorders are known, including sickle-shaped in sickle anemia or target-shaped in thalassemia);
- level of progenitor cells (reticulocytes - immature red blood cells, weakly saturated with hemoglobin).
REFERENCE! The fetal form of hemoglobin is of certain diagnostic interest. Normally, it is present only in the fetus and newborns. As independent breathing and the circulatory system develop, fetal hemoglobin is replaced by normal adult hemoglobin (the amount of fetal hemoglobin in the blood should not exceed 0.5-1.5%).
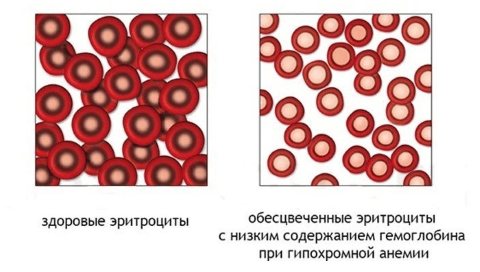
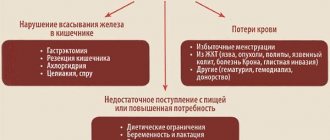
The most common type of anemia is iron deficiency. In this case, tests are carried out to determine the level of iron content:
- serum iron (normal: 11.64–30.43 µmol/l – in men, 8.95–30.43 µmol/l – in women);
- total iron-binding capacity of serum, TIBC (normal: 40.6 – 62.5 µmol/l);
- ferritin (a protein complex that acts as a depot of iron ions);
- transferrin (transport protein) - the norm is 2.0 - 3.8 g/l for men and 1.85 - 4.05 g/l for women.
REFERENCE! Various types of poisoning affect the ability of hemoglobin to carry oxygen, changing its structure (carboxyhemoglobin, methemoglobin are not able to attach oxygen). To identify such deviations, a series of special tests are used. Specific forms of tests are used if B12-deficiency and hemolytic forms of anemia are suspected (analysis for autoimmune reactions).
Hemoglobin, its functions and physiology
Hemoglobin is a complex protein, chromoprotein, respiratory pigment in the blood of humans, vertebrates and some invertebrates. The main function of hemoglobin is to transport oxygen from the respiratory organs to the tissues.
Chemically, hemoglobin belongs to the group of chromoproteins. The hemoglobin molecule consists of a protein part - globin and a prosthetic group of non-protein nature - heme, which includes iron. One hemoglobin molecule contains 4 prosthetic groups. 100 ml of blood from a healthy person contains 13–16 g of hemoglobin. The iron contained in heme is capable of forming a disintegrating compound with oxygen molecules when an erythrocyte passes through the capillaries of the lungs, and when passing through the vessels of other organs, it gives up oxygen and binds to carbon dioxide, which the heme then gives up when the erythrocyte again enters the capillaries of the lungs. The blood flowing through the arteries is saturated with oxygen and has a bright scarlet color; after oxygen is absorbed by the tissues and hemoglobin binds to carbon dioxide, the blood becomes dark red (this blood flows through the veins). In addition to blood hemoglobin, a number of animals have muscle hemoglobin (myoglobin) in rhythmically working muscles with intense metabolism (heart muscle), which is similar in composition and properties to blood hemoglobin.
The alpha polypeptide chain ends with the amino acid combination valine-leucine, and the beta polypeptide chain ends with the combination valine-histidine-leucine. The alpha and beta polypeptide chains in the hemoglobin molecule are not arranged linearly; this is the primary structure. Due to the existence of intramolecular forces, polypeptide chains are twisted in the form of an alpha-helix helix (secondary structure) typical of proteins. The alpha-helix helix itself bends spatially for each alpha and beta polypeptide chain, forming plexuses of an ovoid shape (tertiary structure). Individual parts of the alpha-helix helices of polypeptide chains are marked with Latin letters from A to H. All four tertiary curved alpha and beta polypeptide chains are located spatially in a certain ratio - a quaternary structure. They are connected not by real chemical bonds, but by intermolecular forces.
A Svedberg unit is a complex composed of one heme and one alpha and beta polypeptide chain. Apparently the hemoglobin molecule consists of four Svedberg units. The molecular weight of hemoglobin is 64458, i.e. there are 16115 per iron atom.
In addition to the coordination bond that exists between the polypeptide chains of globin, the Fe++ heme atom has three more coordination bonds, two of which are connected by two nitrogen atoms of the porphyrin ring, and the third, in an environment with low partial pressure of oxygen, is connected to one water molecule. In an environment with high partial pressure of oxygen (arterial blood), the third coordination bond is connected to one oxygen molecule, and the resulting compound is oxyhemoglobin. By continuously converting oxyhemoglobin into reduced hemoglobin and back, oxygen is transferred from the lungs to the tissues. A particularly significant difference between hemoglobin and myoglobin is the oxygen saturation curve, which has a sigmoid shape. This means that the ability of hemoglobin to bind oxygen depends on whether there are other oxygen molecules in this tetramer. If contained, subsequent oxygen molecules attach more easily. Thus, hemoglobin is characterized by cooperative binding kinetics, due to which it combines the maximum amount of oxygen in the lungs and releases the maximum amount of oxygen at those partial oxygen pressures that occur in peripheral tissues.
The P50 value—the value of the partial pressure of oxygen—characterizes the affinity of hemoglobins for oxygen. P50 varies significantly among different organisms, but in all cases it exceeds the value of the partial pressure of oxygen in the peripheral tissues of the organism in question.
This is shown by human fetal hemoglobin (HBF). For HbA P50 26 mm. rt. Art., and for HbF P50 20 mm. rt. Art. Due to this difference, hemoglobin F takes oxygen from HbA found in the placental blood. However, after the baby is born, HbF loses its function; Having a higher affinity for oxygen, it releases less of it in the tissues.
Hemoglobin has another important function: it accelerates the transport of carbon dioxide from tissues to the lungs. Hemoglobin binds carbon dioxide immediately after releasing oxygen; approximately 15% of the carbon dioxide present in the blood is carried by hemoglobin molecules. Carbonic anhydrase, located in erythrocytes, catalyzes the conversion of carbon dioxide coming from tissues into carbonic acid. Carbonic acid quickly dissociates into a bicarbonate ion and a proton, and the equilibrium is shifted towards dissociation. To prevent dangerous increases in blood acidity, there must be a buffer system capable of absorbing excess protons. Hemoglobin binds two protons for every four oxygen molecules released, determining the buffering capacity of the blood.
In the lungs, the opposite process occurs: the addition of oxygen to deoxyhemoglobin is accompanied by the release of protons, which bind to bicarbonate ions, converting them into carbonic acid. Next, effective carbonic anhydrase catalyzes the conversion of carbonic acid into carbon dioxide exhaled from the lungs. Consequently, the binding of oxygen is closely associated with the exhalation of carbon dioxide. This phenomenon is called the Bohr effect.
This effect is a property of tetrameric hemoglobin, which is determined by the heme-heme interaction that underlies the cooperative effects. Protons responsible for the Bohr effect are released as a result of the destruction of salt bridges, which is accompanied by the binding of oxygen to the T-structure; they are detached from the nitrogen atoms of histidine residues (146) in the beta chains. These protons shift the ratio towards the formation of carbonic acid, which is broken down by carbonic anhydrase to form carbon dioxide. On the contrary, when oxygen is released, the T-structure with its inherent salt bridges is formed again, during the creation of which protons are added to histidine residues in the beta chains. Thus, in peripheral tissues, protons favor the formation of salt bridges. The formation of salt bridges causes the release of oxygen from the oxygenated R form of hemoglobin.
Therefore, an increase in proton concentration promotes the release of oxygen, and an increase in oxygen concentration stimulates the release of protons. The first result is expressed in a shift of the oxygen dissociation curve to the right with increasing concentration of hydrogen ions (protons).
The usual hemoglobin concentration in an adult ranges from 80 to 115%, with 100% taken as the average value. Typical values for men are approximately 10% higher than for women. In a child, the normal concentration of hemoglobin differs significantly from the norms of an adult.
Nowadays, there are many ways to find hemoglobin concentration. These include colorimetric methods in which hemoglobin is colorimeterized as oxyhemoglobin or reduced hemoglobin. This group can also include the first method for determining hemoglobin, proposed by Welker in 1854 and modified by Thalquist, the essence of which was that the color of a drop of blood on filter paper was compared with a series of colored paper standards. Another researcher, based on the conversion of hemoglobin into hematin hydrochloride and the associated changes in electrical conductivity, proposed an electronic method for determining the concentration of hemoglobin. There are also gasometric methods. In this case, hemoglobin is saturated with gas (for example, oxygen), carbon monoxide (CO). The amount of hemoglobin is determined by the proportion of absorbed gas. The proportion of oxygen is determined using a Van Slyke device, a Barcroft device, or some other apparatus for determining oxygen. There are methods based on the determination of iron in the hemoglobin molecule. Since the hemoglobin molecule contains a precisely defined amount of iron (0.0347%), the amount of hemoglobin is determined by its amount.
There is such a thing as methemoglobin. Methemoglobin is a derivative of hemoglobin in which the divalent iron atom is converted into a trivalent one. In erythrocytes, during metabolism, certain amounts of methemoglobin are always formed, which is reduced back to hemoglobin under the influence of the enzyme methemoglobin reductase, so that in the whole blood of a healthy person, methemoglobin does not exceed 2% of the total hemoglobin content (0.03 - 0.3%).
The chemical structure of sulfohemoglobin has not been elucidated. It is likely that the two vinyl groups of hemoglobin are connected via SO2 bridges to adjacent methine bonds. Under normal conditions, there is no sulfohemoglobin in the blood. It appears in case of poisoning with antimony compounds, phenacytin, bromine, sulfonamides, nitrates (well water), sulfur compounds, etc.
Determination of sulfohemoglobin in blood can be done spectroscopically. The sulfohemoglobin spectrum does not change with the addition of ammonium sulfide, but disappears with the addition of Na2S2O4 and 2 ml of 10% sodium hydroxide, or a few drops of 3% hydrogen peroxide.
Types of hemoglobin. Recently it was still believed that adult hemoglobin was a single compound. What was known was that in embryonic life there is a special type of hemoglobin, called HbF, which is 155 times more resistant to n/12 sodium alkali than normal hemoglobin. Recently, thanks to the work of Pauling, his colleagues and others, it has become clear that the hemoglobin of an adult, both under normal and pathological conditions, is not a homogeneous chemical compound. Many normal and pathological types of hemoglobin were discovered, which presented hemoglobin metabolism in a new light and indicated ways for studying the pathogenesis of some anemias. It was found that in some diseases there are special types of hemoglobin characteristic of this anemia. Hemoglobin types are of great importance not only for diagnosis, but also transfer the question of the pathogenesis of anemia from a purely morphological area to a biochemical one. Anemia caused by the appearance of a pathological type of hemoglobin is called hemoglobinopathies or hemoglobinoses.
It turned out that humans have three main types of normal hemoglobin: embryonic - U, fetal - F and adult hemoglobin - A. HbU (named after the initial letter of the word uterus) is found in the embryo between 7 and 12 weeks of life, then it disappears and appears fetal hemoglobin, which after the third month is the main hemoglobin of the fetus. Following this, ordinary adult hemoglobin gradually appears, called HbA, after the initial letter of the English word “adult”. The amount of fetal hemoglobin gradually decreases, so that at the moment of birth, 80% of the hemoglobin is HbA and only 20% is HbF. After birth, fetal hemoglobin continues to decrease and by 2–3 years of life it is only 1–2%. The same amount of fetal hemoglobin is in an adult. An amount of HbF exceeding 2% is considered pathological for an adult and for children over 3 years of age.
In addition to normal types of hemoglobin, over 50 pathological variants are currently known. They were first named with Latin letters. The letter B is absent in the designation of hemoglobin types, since it originally designated HbS.
It soon became clear that the letters of the alphabet were not enough to designate all pathological types of hemoglobin. Therefore, they began to use the names of patients, hospitals, laboratories, names of places and districts for this. The most convenient is the nomenclature based on the structural formula.
Both normal and pathological types of hemoglobin differ not in the structure of the protoporphyrin ring, but in the construction of the globin. The difference may be a change in entire pairs of polypeptide chains in the hemoglobin molecule.
This possibility occurs in hemoglobins H, F, Barts, A2 and U. Instead of the normal structure of hemoglobin A - alpha alpha / beta beta (alpha 2 / beta 2), hemoglobin H has a beta beta beta beta structure (beta 4 ), which means that both alpha polypeptide chains are replaced by new beta polypeptide chains. Hemoglobins F, Barts and A2 have two new chains called gamma and delta, and hemoglobin U has a new chain called upsilon. The structure of HbF is alpha alpha/gamma gamma (alpha 2/gamma 2), the structure of hemoglobin Barts is gamma gamma gamma gamma (gamma 4), the structure of HbA2 is alpha alpha/delta delta (alpha 2/gamma 2 ), the structure of hemoglobin U is alpha-alpha/upsilon-upsilon (alpha 2/upsilon 2).
Pathological hemoglobins, which consist of four identical polypeptide chains, are designated tetramers. Alpha and delta tetramers have not yet been observed in vivo.
There is another possibility that occurs in most types of hemoglobin. So, for example, the only difference between HbS and HbA is that in the 6th place in the beta polypeptide chain, instead of glutamine, there is valine, the only difference between HbI and HbA is that in the 16th place in the alpha polypeptide chain is lysine replaced by aspartic acid.
When the anomaly consists of the substitution of an amino acid in the alpha polypeptide chain, then we speak of an alpha anomaly; when it consists of a beta polypeptide chain, we speak of a beta chain anomaly; when in a gamma polypeptide chain, we speak of a gamma chain anomaly (pathological variants of HbF) , when in the delta chain - about a delta chain anomaly (pathological variants of HbA2).
When studying hemoglobin types, the question of the structure of globins is of great importance. On the one hand, the structure is the surest way to differentiate individual types of hemoglobin from one another, on the other hand, it creates the opportunity to compile a strictly scientific nomenclature of the latter. Methods for differentiating types of hemoglobin
To distinguish between individual types of human hemoglobin, electrophoresis is used on a starch block, on starch gel, on agar gel, on cellulose acetate sheets, on acrylamide gel, on carboxymethylcellulose gel, and electrophoresis at high voltage.
The second most important method currently used to differentiate individual types of hemoglobin is chromatography.
Particularly good results are obtained when using Amberlite ion exchange resin and ion exchange dextran gel as an adsorbent.
To distinguish between certain types of hemoglobin, their solubility in certain solvents is also used.
The most famous test in this group is the Itano test to prove the presence of HbS. With this test, reduced HbS is precipitated in 24 m buffer, in contrast to other types of hemoglobin. This test is of particular importance for differentiating HbS and HbD, because HbS and HbD have the same electrophoretic and chromatographic mobility.
To distinguish HbA from HbF, they use, as emphasized above, stability during denaturation with sodium alkali solutions. This is the historically known method by which Kerber differentiated HbA and HbF in 1886.
Group F hemoglobins differ from other hemoglobin types in their characteristic tryptophan band at 289.8 nm in the ultraviolet spectrum. Hemoglobins with group M do not have an absorption band at a wavelength of 630 nm, but show increased absorption at 600 nm.
"Fingerprint method". The case concerns the most important method of establishing the “primary structure” of hemoglobin for various hemoglobin types. The hemoglobin under study is hydrolyzed with trypsin, and the polypeptide chains of the globin molecule are broken down into a large number of peptides. The peptide mixture is subjected to electrochromatography on paper, i.e., electrophoretic separation is carried out in one direction and chromatographic separation in the other. Electrochromatograms characteristic of individual types of hemoglobins are obtained, by which they can be accurately distinguished. Determination of the amino acid composition of individual peptides makes it possible to identify the primary structure of globin of the corresponding hemoglobin type. Drawing an analogy with a forensic technique of similar complexity and accuracy for studying fingerprints, it was called the “fingerprint” method.
The so-called “recombination” or “hybridization” method can be used to determine the composition of polypeptide chains in any hemoglobin type. If you mix known and unknown hemoglobin at pH 4.3, they dissociate into half-molecules consisting of corresponding pairs of polypeptide chains. Polypeptide pairs are again combined into whole hemoglobin molecules after neutralization of the solution, and new “hybrid” hemoglobin molecules can also be obtained. Their identification by electrophoresis or chromatography will allow us to draw a conclusion about the polypeptide structure of an unknown hemoglobin type. This method is also intended primarily for scientific research purposes.
There are also methods for cytologically determining the type of hemoglobin in red blood cells on a blood smear. The presence of HbF in erythrocytes can be substantiated by treating a blood smear with a citric acid buffer mixture with a pH of 3.2 - 3.6. Under these conditions, HbA is removed, and the erythrocytes in which it predominated remain only in the form of erythrocyte shadows, while HbF is retained, and the erythrocytes containing predominantly this type of hemoglobin retain their content.
In addition to all these methods, differences in crystal structure, isoelectric point, etc. are also used to differentiate certain types of hemoglobin.
Consider hemoglobin S, in which the Glu A2 beta residue is replaced by Val.
It is located on the surface of the hemoglobin molecule and comes into contact with water, and the replacement of the polar Glu residue with the nonpolar Val leads to the appearance of a “sticky patch” on the surface of the beta subunit. This sticky region is present in both oxygenated and deoxygenated hemoglobin S, but is absent in hemoglobin A.
There is a complementary region on the surface of deoxygenated hemoglobin that is able to bind tightly to the sticky region of the beta subunit, whereas in oxygenated hemoglobin this region is masked by other groups. When hemoglobin S becomes deoxygenated, its sticky site binds to the complementary site on another deoxygenated hemoglobin molecule, thereby polymerizing deoxyhemoglobin S and depositing it in the form of long fibers.
Deoxyhemoglobin S fibers mechanically deform the red blood cell into a sickle shape, leading to cell lysis and a variety of secondary clinical manifestations.
Therefore, if it were possible to maintain hemoglobin S in an oxygenated state, or at least minimize the concentration of deoxygenated hemoglobin S, it would be possible to prevent the polymerization of deoxygenated hemoglobin S and the formation of sickle cells. The T-form of hemoglobin S is susceptible to polymerization. In sickle cell anemia: hemoglobin S in the ferri state (methemoglobin S) is not subject to polymerization, since it is stabilized in the R-form.
Deoxyhemoglobin A also has a receptor site that can interact with the sticky region of oxygenated or deoxygenated hemoglobin S, but the addition of “sticky” hemoglobin S to deoxyhemoglobin A is not enough to create a polymer, since deoxyhemoglobin A itself does not contain and cannot combine the following sticky region hemoglobin molecule.
This means that the binding of deoxyhemoglobin A to the R- or T-form of hemoglobin S blocks polymerization.
Spiral fibrillar structures are formed as a result of the polymerization of deoxyhemoglobin S. In this case, each hemoglobin molecule comes into contact with four neighboring molecules. The creation of such tubular fibers is responsible for mechanical disturbances in the erythrocyte containing them.
There is another group of pathologies - thalassemia, which are associated with hemoglobin abnormalities. Their characteristics include a reduced rate of synthesis of hemoglobin alpha chains (alpha thalassemia) or beta chains (beta thalassemia). This leads to anemia, which can be very severe.
Nowadays, many studies are being carried out that make it possible to elucidate the molecular devices responsible for the development of thalassemia. Hemoglobin is a complex protein in red blood cells, consisting of 2 parts: protein (globin) and an iron compound (heme). It is iron atoms (heme) that make blood red.
Hemoglobin is involved in the transport of oxygen and carbon dioxide between the lungs and the cells of other organs and maintains the pH of the blood. If there is a lack of hemoglobin in the blood, the transfer of oxygen by hemoglobin becomes difficult. As a result, cells do not receive enough oxygen and their metabolism and functions are disrupted.
Forms of hemoglobin. When glucose is added to the hemoglobin protein (globin), glycosylated (glycated) hemoglobin is formed.
An increase in the level of glycosylated hemoglobin occurs when there is an excess of glucose in the blood, which occurs in diabetes mellitus.
In accordance with the recommendations of the World Health Organization (WHO), analysis of glycosylated hemoglobin is the most effective and necessary method in diagnosing diabetes mellitus. Patients with diabetes are recommended to take a biochemical blood test for glycosylated hemoglobin at least once a quarter.
Doctors distinguish another form of hemoglobin - fetal hemoglobin, which differs from normal hemoglobin in structure and properties. Fetal hemoglobin is the hemoglobin of newborns; the content of fetal hemoglobin in a child’s blood reaches 80%. By the age of 1 year, fetal hemoglobin in children begins to break down and is almost completely replaced by the hemoglobin of adults. Fetal hemoglobin is normal in children, but for adults its content is a sign of serious diseases. Determination of hemoglobin in newborns is used in the diagnosis of blood diseases and cancer.
Hemoglobin norm
| Hemoglobin norm, g/l | |
| Men | 135—160 |
| Women | 120—140 |
The hemoglobin content in the blood of men is higher than that of women. Hemoglobin in a child under 1 year of age is reduced.
The norm of glycated hemoglobin is 4-6.5% of the level of free hemoglobin in the blood. Moreover, the level of glycated hemoglobin may not depend on the concentration of hemoglobin in the blood. The norm of newborn hemoglobin (fetal hemoglobin) in the blood of an adult is up to 1%.
A blood test for hemoglobin is a necessary step in the diagnosis of various diseases. Based on the results of only one blood test for hemoglobin, it is impossible to make an accurate diagnosis, but determining hemoglobin will reveal possible disturbances in the body’s functioning and indicate the need for additional examination.
Increased hemoglobin is a symptom of the following diseases:
Erythrocytosis (a disease accompanied by an increase in the number of red blood cells in the blood)
blood thickening
Congenital heart defects
· intestinal obstruction
· burns
Cardiopulmonary failure.
High levels of glycated hemoglobin are a symptom of diabetes and iron deficiency.
An increase in hemoglobin in the blood occurs after physical exercise, in climbers, and in pilots after high-altitude flights. High hemoglobin in the blood is typical for residents of high mountains. An increased level of hemoglobin in the blood can occur even after being in the fresh air.
The condition of the body in which there is a decrease in hemoglobin in the blood is called anemia. Anemia can develop as a result of loss of hemoglobin during bleeding or blood diseases accompanied by the destruction of red blood cells. Low hemoglobin occurs due to blood transfusion.
The cause of a decrease in hemoglobin - anemia can be a lack of iron or vitamins (B12, folic acid), necessary for the synthesis of hemoglobin and red blood cells.
A blood test for hemoglobin may show low hemoglobin due to various chronic diseases (thalassemia, etc.).
A decrease in the level of glycated hemoglobin occurs with hypoglycemia, hemolytic anemia, bleeding and blood transfusion.
Often there is a decrease in hemoglobin in pregnant women. During pregnancy, hemoglobin usually decreases when there is a lack of iron, since the daily iron requirement of pregnant women increases. If a person usually needs 5-15 mg of iron per day, then a pregnant woman will need 15-18 mg. Doctors recommend that pregnant women monitor the level of hemoglobin in their blood, since low hemoglobin in pregnant women can adversely affect the health of the expectant mother, cause premature birth or fetal growth retardation.
Information about the work “Hemoglobin, its functions and physiology”
Section: Medicine, health Number of characters with spaces: 24765 Number of tables: 1 Number of images: 0
Similar works
Physiology
119359
2
0
... GENERAL PHYSIOLOGY OF EXCITABLE TISSUE (two lessons) Lesson 1 THE NATURE OF EXCITATION 1. What is called irritability and excitability? 2.What is the relationship between the concepts of irritability and excitability? In physiology, which tissues are called excitable and which are non-excitable? 3.What tissues of the body are excitable and non-excitable? 4. Define the concept of “stimulant”. 5.Name two types...
Subject, tasks and methods of age physiology
179193
1
0
...increases. Educational influences aimed at improving internal inhibition play a significant role in this. Literature 1. Badalyan L.O. Neuropathology. – M.: Academy, 2000. – 384 p. 2. Belyaev N.G. Age physiology. – Stavropol: SSU, 1999. – 103 p. 3. Dubrovskaya N.V. Psychophysiology of the child. – M.: Vlados, 2000. – 200 p. 4. Obreimova N.I., Petrukhin A.S. ...
Human physiology
488745
0
0
… . I.P. Pavlov, the first Russian scientist, was awarded the Nobel Prize on October 7, 1904 in recognition of his work on the physiology of digestion. The body's need for food manifests itself in the form of a physiological reaction of hunger. In humans, hunger takes on a pronounced subjective coloring - from relative indifference to food to a strong emotional reaction. The physiological basis of hunger...
Weapons of genocide: human suicide and its mechanisms
440441
3
7
... able to continue to live, despite the instructions of conscience about the wrongness of life...". With this thesis we ended the previous chapter of the book. Without developing it, since this is not included in the rather narrow stated topic of weapons of genocide, let us turn to the internal mechanisms that turn on in people after taking psychotropics. We know what people are running away from (their conscience). But what are people chasing? ...
Biological role of hemoglobin
The main biological role of hemoglobin is to ensure tissue respiration, transport oxygen for energy production and remove carbon dioxide. Moreover, its first function is fundamental for the body, since without hemoglobin oxygen supply is impossible. There are other ways to remove carbon dioxide - 80% of it simply dissolves in the blood and only 20% is carried by hemoglobin.
Disruption of these processes occurs with a decrease in the absolute number of hemoglobin or loss of its activity. To check the sufficiency of oxygen supply to cells, a general blood test is prescribed, and biochemistry shows the consequences of the deficiency:
- oxygen tension;
- oxygen capacity;
- arteriovenous difference in oxygen;
- saturation of hemoglobin with oxygen.
MCNS. Norm
Normal values for the average hemoglobin concentration in erythrocytes are presented in the table.
| Age, gender | Average hemoglobin concentration in erythrocytes g/dl | |
| 1 – 14 days | 28 – 35 | |
| 2 – 4.3 weeks | 28 – 36 | |
| 4.3 – 8.6 weeks | 28 – 35 | |
| 8.6 weeks – 4 months | 29 – 37 | |
| 4 – 12 months | 32 – 37 | |
| 1 – 3 years | 32 – 38 | |
| 3 years – 12 years | 32 – 37 | |
| 12 – 15 years | AND | 32 – 36 |
| M | 32 – 37 | |
| 15 – 18 years old | AND | 32 – 36 |
| M | 32 – 36 | |
| 18 – 45 years old | AND | 32 – 36 |
| M | 32 – 37 | |
| 45 – 65 years | AND | 31 – 36 |
| M | 32 – 36 | |
| Over 65 years old | AND | 32 – 36 |
| M | 31 – 36 | |
Frequently asked questions about the structure of hemoglobin
What is the valence of iron in hemoglobin? Normally, iron is divalent, but upon oxidation it becomes trivalent, which impairs the transfer of oxygen.
What is the peculiarity of the chemical structure of hemoglobin ? Hemoglobin has a quaternary structure, that is, the protein chains and pigment are first connected into complexes (primary structure), and then these four subunits are held together.
What is native hemoglobin and what is its structure? Native hemoglobin is normal, undamaged; its structure contains heme and globin, connected into 4 complexes.
How many iron atoms are in a hemoglobin molecule? Hemoglobin contains 4 hemes with iron, so one molecule contains 4 atoms of this trace element.
The structure of hemoglobin allows it to attach oxygen, transfer it to cells, and take in carbon dioxide. When it decreases, anemia occurs with symptoms of oxygen starvation.