The hemostatic system, responsible for blood clotting, is one of the most important functional systems of the body. The main pathological condition associated with disturbances in the hemostatic system is thrombophilia. The tendency to increased coagulation and blood clots is a common cause of mortality and disability in many countries. There are various forms of thrombophilia, including hereditary. Thrombophilia is especially dangerous for women of reproductive age, as it can cause serious complications during pregnancy.
Determining the mutation of hemostasis genes will allow the attending physician to establish the causes of the disease and select an effective course of therapy.
The Allele Center for Innovative Biotechnology offers a blood test for mutations in hemostatic genes using several markers. To select the necessary set of studies, it is recommended to consult a geneticist. You can make an appointment with a specialist on the website or by phone.
Testing for mutations in genes of the hemostasis system
Thrombophilia develops as a result of an imbalance in the blood coagulation system, with either an increase in the amount of coagulation factors or a decrease in the amount of anticoagulants. The causes of thrombophilia at the genetic level are polymorphisms in the genes of plasma factors of the blood coagulation system. The presence of mutations in at least two genes increases the risk of thrombosis and related complications. An extended analysis for mutations of hemostasis genes includes 12 indicators:
- F2 Prothrombin (coagulation factor II);
- F5 Leiden mutation (coagulation factor V);
- F7 (coagulation factor VII);
- F13A1 (coagulation factor XIII);
- FGB Fibrinogen (clotting factor I);
- SERPINE1 (PAI-1) Plasminogen activator inhibitor;
- ITGA2 Integrin alpha 2;
- ITGB3 Integrin beta 3;
- PROC Protein C;
- PROS1 Protein S;
- MTHFR; MTR; MTRR—folate cycle gene polymorphisms (additional risk factors);
- AGT; ACE; NOS3 - polymorphisms associated with increased risk of blood pressure (additional risk factors)
Causes of mutations
Genes provide a repair (restoration) mechanism: when a section of DNA is damaged, enzymes in the cells of our body “cut out” it and restore the original version. The breakdown of this mechanism is the essence of gene mutation.
We can inherit mutations in hemostasis, the blood coagulation system, on the paternal or maternal side. There are also so-called mutagenic factors that cause gene polymorphism in a completely healthy person - these are dangerous chemicals, ionizing radiation, work in high temperatures and retroviruses.
Who needs testing for mutations in the hemostatic system
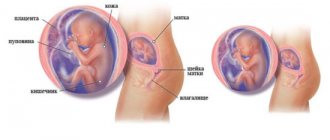
Problems with blood clotting can lead to the development of a large number of pathological conditions. Thrombophilia is the cause of pulmonary embolism, deep vein thrombosis of the lower extremities, myocardial infarction, and stroke. Pregnant women normally experience a physiological increase in blood clotting, so the presence of a hemostasis mutation during this period is more critical and can lead to abruption of a normally located placenta, fetoplacental insufficiency, fetal malformations, recurrent miscarriage, etc.
The following categories of patients are at particular risk:
- patients with a history of stroke, heart attack or thrombosis;
- patients with a family history;
- pregnancy.
The study is also recommended for patients preparing for surgery, as well as people with varicose veins of the lower extremities.
Method for studying genetic material
The laboratory of the Allele Center for Innovative Biotechnologies uses modern equipment and techniques to obtain reliable results. Analysis of genetic samples is carried out using the pyrosequencing method, which accurately determines the presence or absence of polymorphisms in the genes under study.
The research method allows you to confirm or refute the presence of mutations in the genes under study. A double assessment of the test result for mutations in hemostasis genes is excluded with this research method.
Pathology of the hemostatic system and its impact on reproductive function
HEMOSTASIS (BLOOD CLOTTING SYSTEM) IS A PHYSIOLOGICAL PROCESS THAT PROVIDES THE LIQUID STATE OF BLOOD INSIDE THE VASCULAR BED AND AT THE SAME TIME PREVENTS BLOOD LOSS WHEN THE INTEGRITY OF VESSELS IS VIOLATED.
THIS CONTINUOUS PROCESS OF BALANCE BETWEEN THROMBOSIS AND BLEEDING IS CARRIED OUT DUE TO THE INTERACTION AND DYNAMIC EQUILIBRIUM OF VARIOUS CELLULAR AND PLASMA ELEMENTS, AND DISRUPTION OF THIS EQUILIBRIUM LEADS TO EITHER INCREASED BLEEDING OR TO THROMBOSIS. Many studies and discoveries in the field of hemostasiology have helped to reconsider the causes, pathogenesis, concept of treatment and prevention of the most important complications of pregnancy (habitual fetal loss syndrome, premature abruption of a normally located placenta, gestosis, antenatal fetal death, thrombotic complications in pregnant women and in the postpartum period) [5] .
Physiological and pathological changes in the coagulation system
Pregnancy is a special state of the body in which significant changes in hemostasis occur: against the background of hormonal changes, the tone of the vascular walls changes, the potential of the blood coagulation system increases, and the volume of circulating blood increases.
Disruption of this delicate balance in the hemostatic system during pregnancy can lead to a high risk of thrombotic complications, bleeding and obstetric losses [4]. The frequency of these complications increases significantly when carrying genetic defects of the hemostatic system, united by the term “hereditary thrombophilia” (mutation in the prothrombin gene, Leiden mutation, AT III deficiency, protein C and S deficiency, disaggregation thrombocytopathy, etc.), and in acquired forms disorders of hemostasis (hyperhomocysteinemia, APS, SLE, metabolic syndrome, varicose veins, etc.) [4]. Relatively recently, the role of thrombophilia in the pathogenesis of the development of disorders of implantation of the fertilized egg, disturbances in the formation of uteroplacental blood flow and, accordingly, in the formation of gestosis (“toxicosis”) in pregnant women began to be studied. Thus, the presence of thrombophilia poses a serious risk not only for the outcome of pregnancy, but also poses a threat to the life and health of the pregnant woman and the fetus [2]. Clinical manifestations of thrombophilia during pregnancy are very diverse. But timely detection, diagnosis (the complex includes studies such as genetic analysis, homocysteine, antiphospholipid antibodies, assessment of the functional state of platelets, coagulogram) and correct interpretation of the anamnesis and indicators make it possible to diagnose the presence of thrombophilia and, accordingly, begin treatment on time to prevent complications ( both obstetric and thrombotic) [2].
Clinical example 1:
“Patient A. 28 years old. Diagnosis: hereditary predisposition to thrombophilia (heterozygotes F2, F13, ITGB3, FGB), history of thrombosis (PE after cesarean section (CS) 2012). I came in early in my pregnancy. Management of pregnancy on low molecular weight heparins (LMWH), antiplatelet agents, symptomatic therapy. Monitoring of hemostasis indicators, Doppler. Correction of therapy according to indicators. Scheduled CS. Prevention of thrombotic complications in the postpartum period (LMWH for 6 weeks)."
Classifications of thrombophilias
There are several classifications of thrombophilias. Thrombophilias are divided into congenital and acquired:
- Congenital (genetic) thrombophilias are associated with a violation of the structure of the genes of certain proteins that belong to the blood coagulation system.
- Acquired, or situational, thrombophilias are a consequence of age-related changes, surgical interventions, injuries, and the use of hormonal drugs.
Thrombophilias are also distinguished between hematogenous and non-hematogenous:
1. HEMATOGENIC:
- caused by a violation of the vascular-platelet link (thrombocytosis, “sticky” platelet syndrome)
- caused by abnormalities or deficiency of physiological anticoagulants (deficiency of antithrombin III, proteins C and S)
- caused by overproduction or anomaly of blood coagulation factors (Leiden mutation, prothrombin gene polymorphism, hyperfibrinogenemia)
- associated with disruption of the fibrinolysis system (increased plasma levels of PAI-1, plasminogen deficiency)
- metabolic (hyperhomocysteinemia)
2. NON-HEMATOGENIC
- hemorheological forms (slowing blood flow in places of narrowing, turbulent blood flow)
- autoimmune and infectious-immune forms (antiphospholipid syndrome)
- Iatrogenic, including medicinal forms (arising from the use of oral contraceptives, HRT, cytostatics, dexamethasone, surgery on the pelvic organs)
Methods for diagnosing thrombophilia
Diagnostic measures include:
- Family history (for hereditary thrombophilias), history of concomitant diseases (for acquired ones), obstetric history (habitual fetal loss syndrome - SSPP, antenatal fetal death, thrombotic complications during pregnancy or in the postpartum period).
- Laboratory test data (detailed coagulogram with determination of the level of coagulation factors and physiological anticoagulants, determination of platelet aggregation ability, diagnosis of polymorphisms of thrombogenicity genes and the folate cycle using the PCR method).
- Instrumental methods (ultrasound examination of blood vessels, including assessment of the state of uterine blood flow during pregnancy planning and uteroplacental blood flow during pregnancy, duplex scanning of blood vessels, computed and magnetic resonance imaging, echocardiography, etc.).
It is important to timely identify and diagnose other risk factors for complicated pregnancy, which can aggravate the manifestations of thrombophilia and worsen the prognosis.
These include infections, immune and endocrine factors, chromosomal abnormalities, and liver diseases [3]. Clinical example 2:
“Patient D., 42 years old. D-z: hereditary predisposition to thrombophilia (heterozygotes F5, PAI-1, ITGA2, MTHFR677), SPPP, age factor, latent iron deficiency. History: childbirth – 2, missed early pregnancies – 3. Seen after ST. Restorative therapy, additional examination, and preconception preparation were carried out. When pregnancy occurs, therapy is continued: LMWH, folic acid, antioxidants, iron supplements. Urgent birth. Prevention of thrombotic complications in the postpartum period (LMWH for 6 weeks)."
Timely identification of the risks of thrombotic complications, risks of pathology and the threat of pregnancy allows the correct development of effective methods of prevention and treatment. With this approach, therapy acts on the underlying cause of the disease, and not only relieves symptoms [1].
Basic principles of therapy
If a genetic predisposition to thrombophilia, antiphospholipid syndrome, hyperhomocystemia and other combined forms of thrombophilic risks are identified during examination, then successful treatment and prevention of recurrent complications are possible provided that preparation begins early (pre-gravid) (since the pathogenetic negative effects of thrombophilia begin to occur from the moment of implantation, invasion trophoblast and placentation), effects on pathogenesis (anticoagulant, antiplatelet therapy), as well as a differentiated individual approach to each patient depending on the form of thrombophilia and concomitant pathology [1].
Clinical example 3:
“Patient V., 33, D-z: hereditary predisposition to thrombophilia (pathological homozygote PAI-1, heterozygotes integrin a2, b3, FGB), primary infertility, combined factor (IVF - 1 negative result, two cryopreservations - negative result). Ultrasound with color Doppler mapping (CDC): endometrial hypoplasia, impaired hemodynamics of uterine blood flow. I contacted her to plan cryotransfer. Preparation for cryotransfer (LMWH, antiplatelet agents, antioxidants). Indicators during treatment with positive dynamics. Cryotransfer was performed. Pregnancy obtained (bichorionic, biamniotic twins). Management of pregnancy on LMWH, monitoring of hemostasis indicators. Correction of prescriptions according to indicators (At 5-6 weeks - retrochorial hematoma (RCH), at 20-21 weeks - impaired hemodynamics of the uteroplacental blood flow, grade 1A). Planned CS 35–36 weeks. Prevention of thrombotic complications in the postpartum period (LMWH).”
The basic drugs in the process of preparation, management of pregnancy and the postpartum period, of course, are antithrombotic drugs - low molecular weight heparins, antiplatelet agents, as well as folic acid in an individually selected dosage and, according to indications, auxiliary therapy (iron supplements, antioxidants, progesterone preparations, glucocorticoids) [ 3].
Bibliography:
1. Dobrokhotova Yu. E., Shchegolev A. A., Komrakov V. E., etc.; Ed. Yu. E. Dobrokhotova, A. A. Shchegolev. Thrombotic conditions in obstetric practice. – M.: GEOTAR-Media, 2010.2. Makatsaria A.D., Bitsadze V.O. Thrombophilic conditions in obstetric practice. – M.: RUSSO, 2001. – 704 p.3. Mkheidze N. E. Clinical significance of identifying genetic and acquired thrombophilia in pregnant women with a history of premature abruption of a normally located placenta. – M., 2006.4. Repina M. A., Sumskaya G. F., Lapina E. N. Hereditary disorders of the hemostatic system and pregnancy. – St. Petersburg, 2008.5. Physiology and pathology of hemostasis. Ed. Doctor of Medical Sciences prof. N. I. Stuklova. – M.: GEOTAR-Media, 2021.
Laboratory of pathologies of hemostasis in Moscow
You can get tested for genetic mutations of hemostasis at the Experimental Laboratory at the Women's Medical Center on Zemlyanoy Val. The clinic has innovative equipment for PCR diagnostics of genetic thrombophilia and other coagulation tests.
With us you will receive not only test results, but also qualified medical care from the best hemostasiologists in Moscow. Patients with a hereditary risk of thrombosis can count on effective antithrombotic therapy, and women planning to become mothers can receive pregnancy support and full monitoring of health indicators.