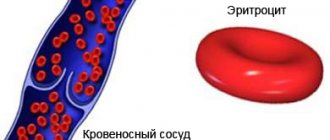
Method of counting red blood cells in the Goryaev chamber
The design of the Goryaev camera and grid . Goryaev's chamber is a thick glass slide cut through four transverse grooves. The grooves divide the glass into plates - two lateral and a middle one. The middle plate is 0.1 mm lower than the side ones. It is divided by a transverse groove into 2 equal parts. A Goryaev grid is applied to each half of the middle plate. The camera is supplied with a ground glass cover glass. It should be applied so that it covers both side and middle plates. By pressing the edges of the glass with your thumbs, it is rubbed against the side plates until rainbow rings (Newton's rings) appear.
Since the side plates are higher than the middle one, a gap remains between it and the cover glass. This is the chamber into which diluted blood is poured. Chamber depth 0.1 mm.
The Goryaev grid applied to the bottom of the chamber is square. Its side is 3 mm, its area is 9 mm.” The grid is divided into 225 large squares—15 horizontally and 15 vertically. Part of the large squares (every two to the third) is divided into 16 small squares. The side of a small square is 1/20 mm.
Counting technique . Correct filling of the counting chamber with diluted blood ensures the accuracy of counting the formed elements. Before filling the chamber, the contents of the test tube are mixed by rotating it between the palms for 2 minutes. The diluted blood is applied to the middle plate of the chamber using a Pasteur pipette with a balloon or a glass rod, placing it near the edge of the coverslip. According to the law of capillarity, liquid flows under the glass, filling the chamber.
After filling, the chamber must be placed on the table for 2-3 minutes, until the movement of liquid in it stops and the cells are positioned motionless against the background of the grid squares. When filling the chamber, liquid should not flow into the grooves. Air bubbles must not enter the chamber.
Counting is carried out under a low magnification of the microscope (magnification 8, approx. 10 or 15), the condenser must be lowered and the diaphragm closed.
Possible errors when counting:
Rules:
- the cells that have more of their half inside it belong to a given square;
— cells divided in half by a boundary line are counted only on the upper and left borders of the square;
— cells that lie with their larger half outside a given square are not counted at all.
Principle . Counting red blood cells under a microscope in a certain number of squares on a counting grid and converting them to 1 µl of blood, based on the volume of squares and blood dilution.
Reagents . 0.9% sodium chloride solution
Special equipment . 1. Goryaev’s counting chamber. 2. Microscope.
Progress of the research . The blood being tested is diluted 200 times. To do this, measure 4 ml of reagent 1 or 2 into a dry test tube. 0.02 ml of blood is drawn with a pipette. The tip of the pipette is wiped with filter paper or gauze, and the blood is blown to the bottom of the tube; the pipette is thoroughly washed in the upper layer of liquid, refilling it and blowing it into a test tube, the contents of the test tube are mixed and left to stand until counting (it is recommended to count red blood cells in the next 2-3 hours after drawing blood, and in hemolytic and 12-deficiency anemias - immediately after collection, as red blood cells may be destroyed). It is unacceptable to leave the taken blood with uncounted red blood cells the next day, since the red blood cells are partially destroyed.
Prepare the counting chamber : wipe the chamber with the mesh and the cover glass dry, then rub the cover glass onto the chamber, lightly pressing on the glass so that rainbow stripes appear along its edges (this indicates the required height of the chamber - 0.1 mm).
Fill the counting chamber with diluted blood : first thoroughly shake the contents of the test tube several times, then use a Pasteur pipette or glass rod to take a drop of diluted blood and bring it to the edge of the cover glass, making sure that it evenly fills the entire surface of the chamber with a mesh without air bubbles. flowing into the grooves. The filled chamber is left in a horizontal position for 1 minute (FOR erythrocyte sedimentation).
To count red blood cells, use a microscope (8x objective, 10x eyepiece), find the upper left edge of the grid. The count is made in 5 large squares, divided into 16 small ones, i.e. in 80 small squares.
Calculation of the number of red blood cells:
produced according to the following formula:
X= a ■ 4000 ■ c
b
where X is the number of formed elements in 1 μl of blood;
a is the number of shaped elements counted into 80 small squares;
b—number of small squares counted;
c — degree of blood dilution; 1\4000 µl - volume of a small square; multiplying by 4000 leads to a volume of 1 µl of blood.
Abbreviated formula: X = a x10000
Standard indicators:
For men: 4.0-5.0 x 10 12
For women: 3.7-4.7 x 10 12
Clinical and diagnostic significance: A decrease in the number of red blood cells in the blood is one of the main laboratory criteria for anemia. However, the degree of erythrocytopenia varies widely in different forms of anemia. Thus, with the most common disease - iron deficiency anemia due to chronic blood loss - the number of erythrocytes can be normal or slightly reduced (3• 106 - 3.6-106 in 1 μl, or 3-10'2 / l - 3.6-10 |2/l). For acute blood loss, B |2
-deficiency anemia (in the relapse stage), hypoplastic anemia, hemolytic anemia (during a crisis), the number of erythrocytes in the blood can decrease significantly and reach I -106 in 1 μl, or 1 • 10|2/l or less.
An increase in the number of red blood cells in the blood - erythrocytosis - can be due to many reasons. Significant erythrocytosis (6.5-106-8.5-10e in 1 μl, or 6.5-10|2/l - 8.5-10|2/l of blood) is one of the important laboratory symptoms of erythremia. Other hemoblastoses of a myeloproliferative nature (subleukemic myelosis or myelofibrosis and chronic myeloid leukemia) are less often accompanied by erythrocytosis and only in the initial stage of the disease.
Secondary (symptomatic) erythrocytosis can accompany a wide range of different diseases and can be absolute (associated with increased normal erythropoiesis) and relative (hemoconcentrating). Absolute erythrocytoses accompany chronic obstructive pulmonary diseases, congenital heart defects, primary pulmonary hypertension, Pickwick's syndrome, hereditary hemoglobinopathies, hypernephroid cancer, cerebellar hemangioblastoma, hepatoma, hormonally active tumors, diseases accompanied by stenosis of the renal arteries, diseases of the central nervous system, etc. Secondary relative erythrocytoses are associated with impaired hemoconcentration and are characterized by a normal volume of circulating red blood cells with a decrease in the mass of circulating blood and the mass of circulating plasma.
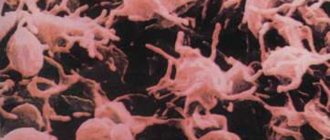
Pathological forms of red blood cells
An increase in the number of red blood cells - polycythemia, can be true (tumor-proliferative process) and symptomatic (reactive irritation of erythropoiesis, blood thickening, impaired nervous regulation and redistribution). A decrease in the number of red blood cells—anemia (posthemorrhagic, hemolytic, due to impaired hematopoiesis).
Qualitative changes in red blood cells
. Anisocytosis is a change in the size of red blood cells (smaller red blood cells are called microcytes; large red blood cells are called macrocytes; red blood cells with a diameter of 12 microns or more are called megalocytes). Poikilocytosis - change in the shape of erythrocytes (pear-shaped, sickle-shaped - drepanocytes, drop-shaped, target-shaped, with elongated ends, schizocytes - fragments of destroyed erythrocytes, acanthocytes - jagged erythrocytes, stomatocytes - erythrocytes with clearing in the center in the form of a narrow linear strip like the shape of a mouth, echinocytes - erythrocytes with multiple outgrowths, anulocytes - erythrocytes in the form of empty rings). Anisochromia is a change in the color intensity of red blood cells in pathological cases. There are pale colored red blood cells - hypochromia, intensely colored - hyperchromia. Red blood cells can be stained not only with acidic, but also with basic dyes - polychromasia.
In pathology, nucleated erythrocytes are found in peripheral blood: normoblasts, erythroblasts and megaloblasts. Megalocytes and megaloblasts are characteristic of Addison-Biermer anemia.
In erythrocytes one can sometimes find remnants of the nuclear membrane - the so-called Cabot rings and nuclear remnants - Jolly bodies in megaloblastic anemia, poisoning with hemolytic poisons, etc. In case of lead poisoning and in people taking blood products, basophilic punctuation of erythrocytes (presence of dotted granularity) is observed. Siderocytes are red blood cells with inclusions of non-hemoglobin iron (ferritin, hemosiderin) in the form of small blue granules.
2.5 White blood cell count
COUNTING THE NUMBER OF ERYTHROCYTES.
The blood contains an average of 4.5-5 x 1012/l (1 µl - 1 mm3 of blood usually contains 4500000-5000000 red blood cells in men, 4000000-4500000 in women.
To count the formed elements, blood taken from a finger is diluted in special mixers (melangers) to create the desired concentration of cells convenient for counting. A counting chamber is used to count the number of red blood cells. Whatever the appearance of the camera, the principle of its design is the same. A grid is located on a glass slide in a recess 0.1 mm high. In Goryaev's chamber (Fig. a), which is most often used, the grid consists of 225 large squares. Of these, 25 squares are further divided into 16 small ones. Each divided square is surrounded on all sides by undivided ones, which makes counting easier (Fig.b) .
To count the formed elements, the blood is diluted to create the desired concentration of cells convenient for counting. To dilute the blood when counting red blood cells, a 3% hypertonic sodium chloride solution is used, in which the red blood cells shrink.
Progress:
1. Before starting work, you need to understand the structure of the counting chamber grid. To do this, place the camera under a microscope and first examine the grid at low and then high magnification, finding small squares and large squares.
2. Accurately measure 4 ml of saline solution into a pre-dried clean conical tube and carefully blow 0.02 ml of capillary blood into it (blood is drawn into the capillary from a Sali hemometer). The result is a dilution of 1:200. The suspension is thoroughly mixed (test tube method by N.M. Nikolaev).
3. Having previously released 1-2 drops out, apply a drop to the chamber mesh, which is previously tightly covered with a cover glass by rubbing. Place a cover glass on the slide in the place where the grid is located on it and carefully press it with your thumbs (Fig. 3). Before the appearance of Newton's rings - rainbow-colored stripes. Release one third of the contents of the mixer onto cotton wool, and blow the next drop onto a glass slide under the cover. Place the slide on the microscope stage and look for the grid at low magnification. Then, with the magnification set to high, count. It is more convenient to count red blood cells at high magnification (eyepiece x 7, objective x 40).
Despite the mixing, the red blood cells are not completely evenly distributed in the field of view. This can be verified by counting the number of red blood cells in several adjacent small squares. To ensure the accuracy of the count, red blood cells are usually counted in 80 small squares (5 large squares located in different places on the grid, for example, diagonally). It is recommended to first draw five large squares on a piece of paper, divide them into 16 small ones, and enter the found number of red blood cells into each small square. To avoid double counting of cells lying on the boundaries between small squares, they are guided by Egorov’s rule: “Red blood cells belonging to a given square are those lying inside the square and on its left and upper border. Red blood cells lying on the right and lower borders in this square are not counted.”
Having calculated so the number of red blood cells (A) in 5 large squares (which is 80 small ones), find the arithmetic average of the number of red blood cells in one small square A/80. The side of one small square is 1/20 mm,
hence its area is 1/400 mm2.
The depth of the chamber is 0.1 mm,
hence the volume of space above one small square will be 1/400 · 1/10 = 1/4000 mm3. If A erythrocytes are found in a volume above 80 small squares, then A/80 per small square. This is in a volume of 1/4000 mm3. Knowing that the volume of the part of the chamber under one small square is equal to 1/4000 mm3, multiply the found number by 4000. The number of red blood cells in 1 μl of diluted blood is obtained. Multiplying by a dilution of 200 gives the number of red blood cells in 1 μl of whole blood. Thus, the formula for calculating the number of red blood cells is as follows:

| X = | A 4000 200 | In 1 ml of blood | X – the required number of red blood cells |
| A – number of red blood cells in 80 small squares | |||
| X = | A 4000 200 106 | In 1 liter of blood |
where X is the required number of red blood cells,
A is the number of red blood cells in 80 small squares.