Full text of the article:
AG - antigen BUT - rapid urease test GM - histological method IPP - proton pump inhibitors ELISA - enzyme immunoassay ICA - immunochromatographic analysis MA - monoclonal antibodies PA - polyclonal antibodies GM - gastric mucosa UDT - urease breath test EGDS - esophagogastroduodenoscopy DOB - delta po compared with the initial value FISH - fluorescence in situ hybridization HP - Helicobacter pylori LCI - narrow-spectrum imaging (linked color imaging) WLE - white-light endoscopy Helicobacter pylori (HP) is one of the most common human infections. The prevalence of this infection in Moscow is 60.7–88% [1, 2], in St. Petersburg – 63.6% [3], in Eastern Siberia it reaches 90% [4, 5]. A meta-analysis published in 2021 suggests that the prevalence of HP remains high in most developing countries, as well as in selected indigenous populations in developed countries, and is generally associated with socioeconomic status and hygiene levels [6]. At the same time, there has been a decrease in the prevalence of HP in developed countries, which is explained by an increase in living standards and improved hygiene [7]. A decrease in the prevalence of HP is associated with a significant decrease in the incidence of gastric cancer and peptic ulcers in Western Europe, the USA and Japan [8]. Current thinking is that HP is transmitted from person to person and causes chronic active gastritis in all infected individuals. This can lead to peptic ulcer disease, atrophic gastritis, gastric adenocarcinoma or gastric MALT lymphoma. Elimination of HP leads to the cure of gastritis, which is the basis for the prevention of long-term complications or relapses of the disease. For these reasons, HP-associated diseases are considered infectious, regardless of symptoms and stage [9, 10]. The substantiation of the pathogenetic role and the eradication of HP led to a fundamental change in the course of peptic ulcer disease, previously a severe recurrent disease [11]. After successfully eliminating the infection, in most cases it is essentially cured. Thus, in Moscow by 2021, compared to 1994, there was a dramatic decrease in the incidence of peptic ulcer disease by 77% (from 167 to 38.6 per 100 thousand population) and the prevalence of this disease by 64% (from 1992 to 717.9 per 100 thousand population). HP eradication has attracted significant interest as a strategy for the primary prevention of gastric cancer, up to 90% of cases of which are caused by HP [12]. Diagnosis and elimination of HP are recommended in patients who are indicated for long-term treatment with aspirin and non-steroidal anti-inflammatory drugs, as well as proton pump inhibitors (PPIs). A test-and-treat strategy is recommended in the presence of dyspepsia symptoms and the absence of anxiety symptoms [9, 10]. The latest international and domestic consensuses recommend eradication therapy for all infected people in the absence of contraindications [9, 10, 13], so information about the advantages and disadvantages of HP diagnostic methods is extremely relevant. Treatment of the infection is possible only after it is identified. Once therapy is completed, its success must be proven. The diagnostic value of most tests decreases with low bacterial contamination, which is observed with current or previous use of antisecretory drugs (PPIs, H2 blockers) and antibacterial drugs, as well as with atrophic gastritis [14]. Adequate interpretation of the results is possible only if PPIs are discontinued 2 weeks, and antibiotics and bismuth preparations are discontinued 4 weeks before the test. Each diagnostic method has its own advantages, disadvantages and limitations. They can be divided into invasive and non-invasive based on the need for esophagogastroduodenoscopy (EGD) to obtain material for research. Due to their high sensitivity and specificity, modern non-invasive tests provide high reliability for detecting HP. The feasibility of performing EGD only for diagnosing HP is questionable; however, biopsy specimens for HP should be taken during diagnostic endoscopy in patients with anxiety symptoms [15] (Table 1). Endoscopy with biopsy of the gastric mucosa (GMU) is justified for bacteriological examination of HP and assessment of sensitivity to antibiotics. Also, methods for diagnosing HP can be divided into direct ones, which determine the pathogen and its genetic material (antigen), and indirect (indirect), which identify metabolic products of the microorganism or antibodies to bacteria in the blood (Table 2).
Bacteriological method
The method is based on identifying the pathogen by culture from a biopsy specimen of the coolant. The biopsy samples obtained during endoscopy are placed in tubes with transport media (Cary-Blaer or Pylori medium). It is advisable to culture the material on the day it is received in the laboratory. Incubation of crops is carried out under microaerophilic conditions with an oxygen content of ≤5%. Subsequently, the isolated cultures are identified, their morphological, tinctorial properties, and sensitivity to antibiotics are determined. The specificity of the method is 98%, sensitivity is 76–90% [16], according to other data – 50–90% [17]. A certain number of false negative results occur when the research methodology is not followed or is not strictly followed. Despite the accuracy of the method, it is rarely used in routine clinical practice due to the duration of the analysis (the average duration of the study is 7 days), the complexity of its implementation and the high cost. The method is recommended after two unsuccessful courses of eradication therapy, when the choice of antibiotics is determined by the sensitivity of HP to them [10, 13].
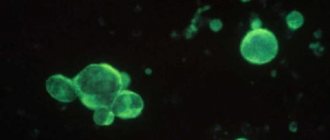
Histological method
GM allows direct visualization of HP and can be recommended for primary diagnosis in patients for whom endoscopy is indicated. The research material is a biopsy specimen of the coolant. The advantages of GM are ease of execution, ease of storage and transportation. The specificity of the histological method can reach 100%, and sensitivity – 91–93% [18]. A highly sensitive (97%) and highly specific (100%) method for diagnosing HP infection is fluorescence in situ hybridization (FISH method) on histological preparations. FISH can identify the coccoid form of HP, which is usually not detected by routine histological examination. In addition, FISH is a rapid, accurate, and cost-effective method for detecting clarithromycin HP resistance in gastric biopsy specimens [15]. Despite the high sensitivity of the histological method, its diagnostic accuracy may be affected by the number of biopsies and the location of their collection. To obtain optimal information, multiple biopsies are recommended, which also provide morphological assessment of the coolant [19]. The Sydney system recommends examining at least five biopsies: two from the antrum (2–3 cm from the pylorus along the greater and lesser curvatures), two from the body of the stomach (along the greater and lesser curvatures, approximately 8 cm from the cardia) and one from corner of the stomach. The value of the study increases when assessing the degree and stage of gastritis using the OLGA or OLGIM system, which allow assessing the prognosis of the disease [20]. If the contamination of HP is low, as well as if bacteria are lost during the preparation process, false negative results are likely. If the histologist is poorly trained, it is possible that other microorganisms may be identified as NR—a false positive result. The sensitivity and specificity of GM in patients with upper gastrointestinal bleeding average only 70% [21]. Therefore, the issue of diagnosing and treating HP should be returned to during the period of remission as planned. It should be taken into account that HP can be detected only on fairly thin and well-stained sections. In the presence of atrophic changes in the coolant, the frequency of false negative results increases. Moreover, in the area of intestinal metaplasia, HP is in most cases not detected by either conventional or special staining methods, despite serological signs of infection. The disappearance of HP correlates with the development of intestinal metaplasia and a decrease in gastric secretion [22]. In patients taking antisecretory drugs (H2-blockers, PPIs), bismuth preparations and antibiotics, the sensitivity and specificity of GM are reduced [19, 22]. A universal recommendation is to discontinue PPIs 2 weeks before the study, antibiotics and bismuth preparations – 30 days [23]. The cytological method is one of the options for histological examination. The material is smear-imprints of biopsy samples of the coolant, taken during endoscopic examination, which are subsequently dried and stained using the Romanovsky-Giemsa method.
Who needs to get tested for Helicobacter?
An examination is necessary if you notice one of the following symptoms:
- discomfort and pain mainly in the upper abdomen
- complaints of bad breath
- recurrent caries
- heartburn
- belching
- stool disorders
- nausea and even vomiting
An additional reason for diagnosing Helicobacter pylori is previous diseases of the gastrointestinal tract (chronic gastritis, gastroduodenitis, peptic ulcer of the stomach and duodenum, surgery for stomach cancer), as well as hereditary predisposition (the presence of bacteria or gastrointestinal diseases in family members ).
Helicobacteriosis is a serious problem, but sometimes a person is not even aware of the presence of bacteria in the body. This is why gastroenterologists at the EXPERT Clinic recommend being tested for Helicobacter Pylori, even for the purpose of prevention.
Who can be infected with Helicobacter?
Up to 80% of the Russian population is infected with the bacterium Helicobacter pylori.
The situation is complicated by the fact that the spread of infection can occur very easily. The bacterium is transmitted during a kiss, when using the same utensils, personal hygiene products (toothbrush), when cleaning a baby’s pacifier by “licking” an infected mother, etc. If one family member is infected, then with a high degree of probability we can say that and other family members will be infected over time.
Rapid urease test
RUT is an indirect method for diagnosing HP based on the detection of urease activity of the bacterium. The test requires taking a biopsy of the coolant, which is placed in a test tube (well) with urea and an indicator on a liquid, gel or dry basis. By changing the color of the indicator when the pH of the medium increases as a result of the breakdown of urea by microbial urease, NR can be identified with fairly high accuracy. In clinical practice, if there are indications for endoscopy and there are no contraindications to biopsy, BUT is recommended to be used as a first-line diagnostic method [24]. The sensitivity of BUT, depending on the number of biopsy samples, varies between 80–90% [26] (according to other data, from 61 to 74% [27]). Increasing the number of biopsy samples and taking them from two parts of the stomach (from the body and antrum) makes it possible to increase the sensitivity of RUT [28]. When using high-quality tests and several gastrobiopsy samples, and good processing of biopsy forceps, the specificity of the test can reach 95–100% [25, 26] (according to other sources - 90% [17]). False-positive results are rare and may be due to the presence of other urease-containing bacteria such as Proteus mirabilis, Citrobacter freundii, Klebsiella pneumoniae, Enterobacter cloacae and Staphylococcus aureus. False-negative test results are observed more often than false-positive ones, which makes it impossible to use a negative result to exclude ADR [10]. False-negative results can be obtained in patients who have suffered ulcer bleeding while taking PPIs, antibiotics, bismuth preparations, as well as in cases of severe atrophy or metaplasia of the gastric mucosa [29]. Thus, a positive RUT result indicates the presence of ADR and makes it possible to prescribe treatment, but a negative result does not allow one to exclude ADR, therefore RUT is not recommended for assessing the effectiveness of eradication [10].
How to prepare for the procedure
The patient is given a special plastic tube, which must be placed in the oral cavity. You just need to breathe for a few minutes - normal breathing, you don’t need to do anything special. Then, at the doctor’s signal, the tube is removed, and you need to drink the so-called urea test solution. Then the tube is inserted into the mouth with the other end and you need to breathe again. The only condition that requires testing for Helicobacter is that saliva does not get inside the tube. The test results are processed on a digital device, which reduces the examination time and guarantees high information content.
13C/14C urease breath test
UDT is also based on the detection of urease activity of NR. In the presence of NR, hydrolysis of a urea solution taken orally, labeled with a carbon isotope (13C or 14C), occurs. Carbon dioxide (13CO2 or 14CO2) enters the blood and is eventually released through the lungs in the exhaled air. Air samples are taken initially and after a certain time (when using 13C-urea - 30 minutes) after taking the test solution. The increase in labeled CO2 is expressed as delta over baseline (DOB). Typically, DOB >2.0‰ is used as a criterion for the presence of HP infection. The DOB value has been shown to positively correlate with the bacterial load of HP [30]. The sensitivity of 13C-UDT is 96%, specificity is 93% [31]. Initially, UDT was developed using urea labeled with the weakly radioactive isotope 14C, but now it has almost completely been replaced by a test with urea labeled with the stable non-radioactive isotope 13C [31]. 13C-UDT is preferred over 14C-UDT because it avoids even minimal exposure to radiation. However, the lower cost of the test and equipment makes 14C-UDT popular in developing countries. The diagnostic accuracy of 13C-UDT and 14C-UDT is no different, and both tests can be considered the “gold standard” among non-invasive methods for diagnosing HP [32]. False-positive results are rare, but can be observed after performing an endoscopy with a biopsy immediately before the test, in patients who have undergone gastrectomy, and also with a significant decrease in gastric secretion. A small number of false negative results may be due to violations of the methodology for taking and storing exhaled air samples, physical activity the day before and during the test. As with most other tests, a reliable UDT result can be obtained after 2 weeks of PPI discontinuation and no earlier than 4 weeks after stopping antibiotics and bismuth preparations. The Maastricht V consensus ranks urea-labeled UDT as the best method for diagnosing HP with high sensitivity and specificity and excellent performance both for the initial diagnosis of infection and for assessing the effectiveness of eradication [10]. In Russia, a breath test with unlabeled urea (“Helic-test”) has become widespread. The results of an open multicenter study of the effectiveness of breath tests demonstrated its insufficient sensitivity (78%) and low specificity (62%) [33]. The authors of another Helic-test study noted that the low specificity of NH3-urease tests and, as a consequence, the high frequency of false-positive results do not allow their use for the primary diagnosis of infection and monitoring the effectiveness of HP eradication [34].
Examination of stool for the presence of HP antigen
Fecal HP antigen (HP antigen) testing is another non-invasive diagnostic method with high sensitivity (94%) and specificity (97%) [35]. There are two research options: enzyme-linked immunosorbent assay (ELISA) and immunochromatographic assay (ICA) using polyclonal (PA) or monoclonal (MA) antibodies. MA-based tests are more accurate and ELISAs provide more reliable results than ICA [35]. Along with 13C-UDT, a monoclonal test based on ELISA is recommended both for the primary diagnosis of HP infection and for monitoring eradication [10]. Monoclonal AG in stool is a convenient and effective test for diagnosing HP in children [36, 37]. In addition, the HP antigen test can be used for epidemiological studies and screening programs due to the relatively low cost of the test and equipment [21]. The reasons for false-negative results may be uneven distribution of antigen in the stool, destruction of antigen when evacuation of feces is slowed down (constipation), and gastrointestinal bleeding [37].
Serological method - detection of IgG antibodies to HP in blood plasma
HP colonization induces a systemic immune response. 3-4 weeks after infection, antibodies to HP appear in the blood of patients. These antibodies are determined by ELISA. Because the infection is chronic and does not clear spontaneously, positive serologic tests in untreated patients indicate ongoing infection. The specificity of the method is 93–94%, sensitivity is 59–71% [38]. Despite the fact that the level of antibodies decreases during successful eradication, the serological reaction remains positive for a number of years. This “serological scar” prevents the use of serological blood testing to evaluate the effectiveness of treatment (cause of false positive results). In addition, the serological method is not very informative in patients with a weak immune response, and IgG antibodies appear no earlier than a month after infection (the reason for false negative results). The advantage of the serological method for the primary diagnosis of HP is the possibility of its use in people taking PPIs and antibiotics [10], as well as after gastrointestinal bleeding and in case of coolant atrophy. All of these situations are associated with a decrease in the bacterial “load”, which is why other diagnostic tests can give a false negative result [39]. There is growing interest in expanding the ability to detect HP infection in real time during endoscopic examination of the stomach, which will reduce the cost of diagnosis and treatment. New opportunities for diagnosing not only precancerous diseases of the stomach, but also HP infection were opened by research in a narrow-spectrum mode. Linked color imaging (LCI) is a new imaging endoscopy technique developed in Tokyo that uses narrow-band, short-wavelength light to detect inflammation or atrophy and precancerous changes in the mucosa. A retrospective study of endoscopic images of 60 patients (30 with proven HP infection and 30 without HP infection) was conducted to compare the accuracy of LCI and traditional white-light endoscopy (WLE) for diagnosing HP infection. Endoscopically detected diffuse hyperemia of the mucous membrane, which could correlate with HP infection, was assessed. The authors found higher accuracy, sensitivity, and specificity (85.8, 93.3, and 78.3%, respectively) for diagnosing HP using LCI compared with conventional WLE (74.2, 81.7, and 66.7%, respectively). respectively). The authors concluded that endoscopy using LCI is more informative than WLE for detecting diffuse hyperemia of the coolant and, accordingly, suspicion of the presence of HP [40]. The main tasks facing the doctor are diagnosing HP, selecting an effective treatment regimen and, subsequently, assessing the effectiveness of the treatment. When choosing a HP testing method, it is necessary, first of all, to take into account its sensitivity and specificity. Each of the methods used today has its drawbacks, and therefore it is undesirable to limit oneself to only one of them in practical activities. Methods that can be used for primary diagnosis are presented in table. 3, to monitor the effectiveness of therapy - in table. 4. Taking PPIs limits the accuracy of diagnostic methods due to a decrease in the urease activity of HP, as well as a decrease in the number of vegetative (spiral) forms of the bacterium. One study comparing the inhibitory effect of PPIs on HP showed that, unlike omeprazole and lansoprazole, pantoprazole did not inhibit HP growth and urease [40]. The diagnostic value of tests after 7 days of PPI withdrawal is being investigated. However, the universal recommendation remains a 14-day withdrawal from all PPIs before most tests. In clinical practice, a situation is often observed when a patient presents with symptoms of dyspepsia, who has not previously been treated with HP, but is currently taking or has recently taken PPIs, H2-blockers, antibacterial or bismuth-containing drugs. In this case, to diagnose HP, it is possible to perform an enzyme immunoassay in blood for antibodies to HP of the IgG class; other diagnostic methods will be uninformative. At the same time, it is known that a 28-day intake of bismuth tripotassium dicitrate can in some cases (up to 33%) lead to the eradication of HP [41], therefore, to make a decision on treating the infection, non-invasive diagnostics (for example, 13C- UDT) no earlier than 30 days after therapy. If the patient has previously been treated for HP and is currently taking or has taken PPIs, H2-blockers, antibacterial or bismuth-containing drugs, then reliable diagnosis of HP is possible no earlier than 30 days after completion of taking antibiotics and bismuth drugs and no earlier than 2 weeks after stopping PPI use. Any test can be used except serology. If there are no indications for EGDS, non-invasive diagnostics is preferable. A summary of the methods for diagnosing HP is presented in Table. 5. Monitoring the effectiveness of HP eradication, regardless of the tests used, should be carried out no earlier than 30 days after completion of all drugs in the regimen. Failure to comply with this rule leads to a false conclusion about the effectiveness of therapy. Preference should be given to non-invasive methods: 13C-UDT and determination of the HP antigen in stool [9, 10]. An exception may be cases that require a repeat endoscopy, in which a biopsy specimen can be obtained for histological, cytological or bacteriological examination. However, control endoscopy in patients with exacerbation of peptic ulcer disease is usually carried out against the background of continued use of PPIs, which makes the diagnosis of HP impossible. Carrying out an endoscopy a month after completion of treatment only for the purpose of detecting infection can be justified only if non-invasive methods are unavailable and impossible to use. However, RUT should not be used to exclude infection [10]. One of the common mistakes is the use of a serological method to monitor the effectiveness of eradication: after successful elimination of HP, antibodies remain in the blood for a long time. However, according to the observational study "PARAD", the serological method to control eradication was used in 17.8% of cases, which is a gross error. In addition, monitoring of the effectiveness of treatment was carried out less than 4 weeks after the end of therapy in 62.3% of cases, which is also a serious deviation from the recommendations [42]. An analysis of data from Russian patients included in the European HP Register (Hp-EuReg) shows that to monitor the effectiveness of therapy, a serological test is used in 2.5–3.6% of cases [43, 44]. The high prevalence and etiopathogenetic connection of HP with the most significant diseases of the stomach dictate the need to optimize the diagnosis of this infection, taking into account the sensitivity and specificity of tests, as well as the conditions under which they are carried out. Before prescribing therapy, the infection must be identified, and after treatment, its success must be confirmed. It is important to emphasize that the lack of assessment of the effectiveness of HP eradication, on the one hand, does not allow documenting the achievement of the goal in a particular patient, and on the other, deprives the doctor of the opportunity to assess the effectiveness of prescribed treatment regimens in a given region. The latter is fundamentally important for critical rethinking and improvement of clinical practice. A wide arsenal of diagnostic tests, when used rationally, allows us to successfully solve these problems.
Why diagnose and treat helicobacteriosis?
Helicobacter pylori promotes the development of:
- stomach cancer
- mucosal atrophy
- peptic ulcer
- non-ulcer dyspepsia
- gastritis.
* % of cases of development of diseases associated with the bacterium Helicobacter Pylori.
The mechanism of the negative impact of Helicobacter Pylori
Helicobacter Pylori is a gram-negative bacterium that infects the stomach and duodenum. The bacterium secretes toxic enzymes that damage the cells of the stomach and duodenum.
This causes ulceration of the mucous membrane. Helicobacter Pylori delays the healing of ulcers, the unprotected wall of the stomach begins to be exposed to gastric juice, and this contributes to the transition of the disease to a chronic form. The bacterium impedes trophism and microcirculation of tissues, which in turn also interferes with the normal healing of any damage. In this case, therapy that is aimed at eradicating bacteria (their complete destruction) and normalizing regeneration mechanisms can help.