Electrolytes in the blood, their normal ratios, are the main condition for muscle contraction of the myocardium, and, consequently, life itself.
When many readers familiar with technology and chemistry hear the word “electrolyte,” the first thing that comes to mind is the liquids contained in the battery, batteries, and other power sources. In fact, electrolytes are found in all living things without exception, since each cell requires the movement of individual particles, leading to metabolism. More advanced compounds, such as proteins and enzymes, are immersed in the cytoplasm, the very basis of which, as well as the intercellular fluid, is an electrolyte.
Electrolytes include the simplest ions known to us from inorganic chemistry and possessing an electrical charge. These ions are capable of creating an electric current, which is based on the entire functioning of the nervous system and sensory organs. They promote the absorption of nutrients, stimulate metabolism and remove metabolic products from the body through the kidneys.
Only thanks to blood electrolytes do the cells contain as much water as needed, and there is a stable acid-base balance in the body. Basic electrolytes transport water molecules from the blood and intercellular fluid into cells and back, they maintain osmotic balance and equality of concentrations in certain proportions, they stimulate or inhibit enzyme systems depending on need. What are the main electrolytes in our body, and what role do they play?
Basic electrolytes and their functions

The main protozoan, positively charged cations are sodium, potassium, which are monovalent, divalent magnesium and calcium cations, and the negatively charged chlorine anion. Their functions are:
- sodium is the main component of extracellular fluid, it retains the required volume of water in the body, the isolation of nerve impulses depends on it, and it is also the main substance that ensures the constancy of the balance of other electrolytes;
- Potassium is the most important component of the intracellular environment. In every living cell there is always more potassium than sodium, which is more abundant outside. It is potassium ions that stimulate any cellular action and impulses. Potassium ions provide electrical signals that are transmitted by nerves. It is potassium ions that trigger each beat of our heart, using a mechanism called spontaneous diastolic depolarization of the cells of the atriosinus node (pacemaker);
- chlorine is a negatively charged monovalent anion, and its main role is to form hydrochloric acid, which is produced in the stomach by parietal cells and takes an active part in digestion, being the main component of gastric juice;
- Magnesium is also necessary for the functioning of the muscular system, for the transmission of nerve impulses, for energy metabolism and for the metabolism of neurons. Magnesium is a calcium antagonist, and prevents the precipitation of its salts into an insoluble precipitate, thus preventing the formation of calcifications in the body;
- Calcium is mainly deposited in the form of phosphates in bone tissue. It is also necessary for proper muscle function, for the absorption of iron, takes part in the work of many enzymes and regulates blood clotting.
Thus, electrolytes work in pairs, being mutual antagonists of each other: sodium and potassium, calcium and magnesium.
Sodium

Sodium is the main extracellular cation, an element that helps the body actively grow and develop. It ensures the transport of nutrients to the cells of the body, participates in the generation of nerve impulses, has an antispasmodic effect, activates digestive enzymes and regulates metabolic processes.
The norm of sodium in the blood for adults is 135 – 150 mmol/l. (For children – 130 – 145 mmol/l).
Sodium leaves the body through sweating. People constantly need it, especially those who experience severe physical activity. You need to constantly replenish your sodium supply. The daily sodium intake is about 550 mg. Plant and animal sources of sodium: table salt, cereals, soy sauce, vegetables, beans, organ meats, seafood, milk, eggs, pickles, sauerkraut.
When the amount of sodium cations in the blood changes, the functioning of the kidneys, nervous system, and blood circulation is disrupted.
A blood test for sodium electrolytes is carried out for gastrointestinal dysfunction, diseases of the excretory system, and endocrine pathologies.
Hypernatremia (increased levels of the element in the blood) develops when:
- Excess salt in the diet,
- Long-term hormone therapy
- Pituitary hyperplasia,
- Adrenal tumors,
- Comatose state
- Endocrinopathies.
The causes of hyponatremia are:
- Refusal of salty foods,
- Dehydration resulting from repeated vomiting or prolonged diarrhea
- Hyperthermia,
- Loading doses of diuretics,
- Hyperglycemia,
- Hyperhidrosis,
- Prolonged shortness of breath
- Hypothyroidism,
- nephrotic syndrome,
- Heart and kidney diseases,
- Polyuria,
- Cirrhosis of the liver.
Hyponatremia is manifested by nausea, vomiting, decreased appetite, palpitations, hypotension, and mental disorders.
Blood test for electrolytes - what is it?
The norms of blood electrolytes are quite narrow in their range, since it is from the concentration of inorganic compounds that the secondary parameters of the main environment of the body are produced, against the background of which all other biochemical processes unfold. The most important of the electrolytes listed are sodium and potassium. If their mutual relationship is disrupted, then the fluid in the body is either retained or leaves. In case of dehydration, the concentration of these ions increases significantly, resulting in disturbances in the functioning of the heart, kidneys, musculoskeletal system and striated muscles, arrhythmia and convulsions appear.
In order to understand that this disorder is caused by a change in the concentration of electrolytes in the blood plasma, these biochemical studies of the concentrations of Na, K, Cl, Mg, Ca are used. What are the indications for studying blood plasma electrolytes? These are the following conditions in which there are electrolyte imbalances:
- profuse diarrhea and vomiting, exposure to hot climates, which leads to severe sweating, serious burns affecting a large area;
- in case of disturbances of acid-base balance - metabolic acidosis and alkalosis;
- when severe swelling occurs;
- in the presence of nagging muscle pain, cramps;
- in the event of extrasystole, atrial fibrillation, and other rhythm disturbances;
- if the patient, especially the elderly, is at risk of overdose of diuretics;
- to monitor the condition of patients with chronic kidney and heart diseases, especially chronic renal and congestive heart failure;
- with lethargy, drowsiness, stupor, stupor, various disorders of consciousness;
- for disorders of mineral metabolism in bones, osteoporosis;
- if the patient has endocrine pathology (hyperparathyroidism, diabetes insipidus).
There are many other indications that the doctor determines in each specific case. What is the normal level of electrolytes in the blood of a healthy adult?
Magnesium
Magnesium is a vital electrolyte that works alone or in conjunction with other cations: potassium and calcium. It normalizes myocardial contraction and improves brain function. Magnesium prevents the development of calculous cholecystitis and urolithiasis. It is taken to prevent stress and cardiac dysfunction.
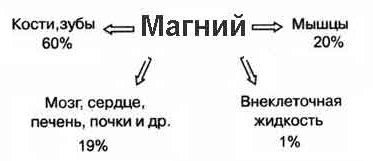
distribution of magnesium ions in the body
The generally accepted norm of magnesium in the blood is 0.65-1 mmol/l. Determination of the amount of magnesium cations in the blood is carried out for patients with neurological disorders, kidney diseases, endocrine pathologies, and rhythm disturbances.
Hypermagnesemia develops when:
- Insufficient amount of thyroid hormones in the blood,
- Pathologies of the kidneys and adrenal glands,
- Dehydration,
- Long-term and uncontrolled use of magnesium-containing drugs.
The causes of hypomagnesemia are:
- Starvation diets,
- Colitis,
- Worms,
- Pancreatitis,
- Thyrotoxicosis,
- Rickets,
- Hereditary phosphorus deficiency,
- Hypercalcemia,
- Alcoholism.
Some foods are sources of magnesium - oatmeal, bran bread, pumpkin seeds, nuts, fish, bananas, cocoa, sesame seeds, potatoes. The absorption of magnesium is impaired by the abuse of alcoholic beverages, frequent use of diuretics, and hormonal drugs.
Norms of blood electrolytes and reasons for deviations from reference values
The table of blood plasma ion balance indicators in the absence of pathology should have the following range of values:
| Element | millimoles per liter, mmol/l |
| potassium | 3,5-5,1 |
| sodium | 136 — 145 |
| chlorine | 98-107 |
| magnesium | 0,66-1,07 |
| calcium | 2,1 – 2,55 |
The indicated electrolyte standards do not include some age-related features that may be required when analyzing children. What are the most common causes of deviations from the norm? Here they are:
Sodium

An upward change in sodium values appears with endocrine pathology, with the consumption of large amounts of salt in food, with long-term use of drugs such as corticosteroid hormones, androgens and estrogens, and in women with oral contraceptives.
A lack of sodium in the human body occurs when there is a lack of salt in food, with profuse diarrhea, sweating and vomiting; the same loss of water and sodium through the skin occurs during fever. Sodium is lost with large doses of diuretics, in diseases such as diabetes and chronic adrenal insufficiency, as well as in the case of severe liver and kidney diseases.
Potassium

For viral hepatitis and destruction of liver tissue, for cytolysis and anemia, for burns, for various types of shocks, for acute renal failure, as well as for effective treatment with chemotherapy when tumors disintegrate. Hypokalemia, or a lack of potassium ions in the blood, occurs with the development of metabolic alkalosis, or excessive alkalization, with diabetes insipidus, with frequent deep breathing.
Chlorine

In the clinic, an excess of chlorine is occasionally encountered, but a deficiency can be detected quite often. It occurs with profuse indomitable vomiting, when all the synthesized chlorine for gastric juice leaves the body, with water poisoning, overhydration and polydipsia, with indomitable thirst, when there is no dehydration.
Also, a lack of chlorine is caused by excessive intake of diuretics, when it is secreted into the urine, in severe traumatic brain injuries, and in metabolic acidosis. Chronic and long-term chlorine deficiency can be accompanied by pathology of skin appendages, baldness and tooth loss.
Our article “The norm of chlorine in the blood and the reasons for elevated levels” is devoted to the content of chlorine in the blood.
Calcium

In this case, calcium is directly absorbed into the blood. An increase in calcium levels is caused by diffuse toxic goiter and thyrotoxicosis, tuberculous bone damage, as well as excess vitamin D. Calcium deficiency is widespread in rickets in children, in menopausal osteoporosis in women associated with estrogen deficiency, in myxedema or hypothyroidism due to chronic pancreatitis, when fat-soluble compounds containing vitamin D2 are not absorbed.
You can read more about the calcium content in the blood in our articles: “Normal calcium in the blood”, “Lack of calcium in the body: symptoms and signs”, “Ionized calcium: role in diagnosis, normal in the blood, reasons for increase and decrease”.
Magnesium
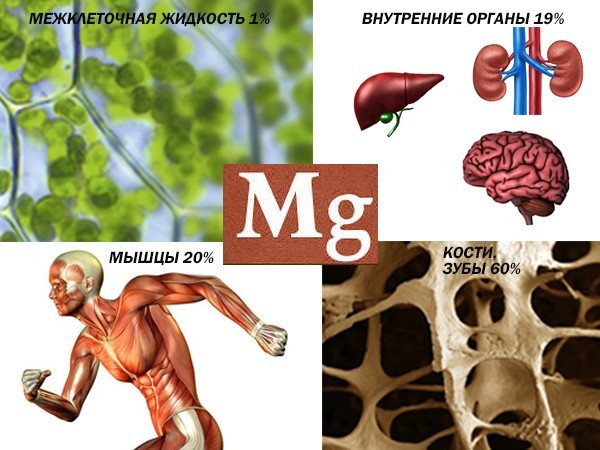
Conditions with increased magnesium are the opposite of calcium deficiency, and vice versa. But the most common are dehydration and taking diuretics, excessive intake of magnesium and antacids (they contain a lot of magnesium).
Its content in the blood decreases with hyperfunction of the thyroid gland, fasting and strict vegetarian mono-diets, intestinal diseases, as well as with chronic alcoholism.
Read more in the articles “Magnesium norm in a blood test: indications for analysis, causes of magnesium deficiency” and “Lack of magnesium in the female body: symptoms, causes, treatment.”
We also invite you to take a short test of 12 questions: Do you have enough magnesium? Test for women.
This short review listed the most important electrolytes in our body. Currently, not a single major operation can be performed without their determination; patients in the intensive care unit undergoing dialysis are regularly tested for the content of electrolytes in the blood. Sometimes, in general outpatient practice, there is also a need to conduct such tests.
Preparing for the study

The study is carried out on an empty stomach
Preparation for analysis allows you to obtain the most accurate research result. The main points in it are:
- refusal to eat 12 hours before blood donation;
- You can drink only clean water without gas in the morning before the analysis;
- exclusion of physical and emotional stress the day before the analysis;
- quit smoking 2 hours before the test.
When taking medications, the doctor must be warned about this so that the specialist, when interpreting the results, can make adjustments for the effect of the drugs.
Calcium

Calcium is an electrolyte responsible for the normal functioning of the coagulation and cardiovascular systems, regulation of metabolism, strengthening the nervous system, building and ensuring the strength of bone tissue, and maintaining a stable heart rhythm.
Hypercalcemia develops when:
- Hyperfunction of the parathyroid glands,
- Cancerous destruction of bones,
- Thyrotoxicosis,
- Tuberculous inflammation of the spine,
- Kidney pathologies,
- Gout,
- Hyperinsulinemia,
- Excessive intake of vitamin D into the body.
The causes of hypocalcemia are:
- Bone formation disorders in children,
- Bone loss,
- Lack of thyroid hormones in the blood,
- Inflammatory and degenerative processes in the pancreas,
- Magnesium deficiency
- Violation of the bile excretion process,
- Liver and kidney dysfunction,
- Long-term use of cytostatics and antiepileptic drugs,
- Cachexia.
The following foods are sources of calcium: milk, white beans, canned tuna, sardines, dried figs, cabbage, almonds, oranges, sesame seeds, seaweed. Sorrel, chocolate, spinach are foods with an antagonistic effect that suppress the effect of calcium. This microelement is absorbed only in the presence of an optimal amount of vitamin D.